1,3,5-Triphenylbenzene and Corannulene as Electron Receptors for Lithium Solvated Electron Solutions
Summary
The authors report on conductivity studies carried out on lithium solvated electron solutions (LiSES) prepared using 1,3,5-triphenylbenzene (TPB) and corannulene as electron receptors.
Abstract
The authors report on conductivity studies carried out on lithium solvated electron solutions (LiSES) prepared using two types of polyaromatic hydrocarbons (PAH), namely 1,3,5-triphenylbenzene and corannulene, as electron receptors. The solid PAHs were first dissolved in tetrahydrofuran (THF) to form a solution. Metallic lithium was then dissolved into these PAH/THF solutions to yield either blue or greenish blue solutions, colors which are indicative of the presence of solvated electrons. Conductivity measurements at ambient temperature carried out on 1,3,5-triphenylbenzene-based LiSES, denoted by LixTPB(THF)24.7 (x = 1, 2, 3, 4), showed an increase of conductivity with increase of Li:PAH ratio from x = 1 to 2. However, the conductivity gradually decreased upon further increasing the ratio. Indeed the conductivity of LixTPB(THF)24.7 for x = 4 is even lower than for x = 1. Such behavior is similar to that of the previously reported LiSES prepared from biphenyl and naphthalene. Conductivity versus temperature measurements on corannulene-based LiSES, denoted by LixCor(THF)247 (x = 1, 2, 3, 4, 5), showed linear relationships with negative slopes, indicating a metallic behavior similar to biphenyl and naphthalene-based LiSES.
Introduction
Lithium solvated electron solutions (LiSES) prepared using simple two-ring polyaromatic hydrocarbons (PAH) such as biphenyl and naphthalene can potentially be utilized as liquid anodes in refuelable lithium cells1-7. In the LiSES, these simple PAH molecules served as the electron receptors for solvated electrons from dissolved metallic lithium.
Progressing from these two-ring systems, the authors have since then carried out conductivity measurement studies on LiSES which are prepared using more complex PAHs, starting with the group of cyclopenta-2,4-dienone derivatives8. These PAHs include larger PAHs (>two benzene rings) and PAHs with substituents incorporated into their aromatic rings. A larger PAH molecule with more than two rings is expected to accommodate more lithium atoms per PAH molecule than either biphenyl or naphthalene thus resulting in LiSES with a higher energy density. The objective of introducing substituents into PAHs is to make the PAH accept electrons more readily and become more stable as polyanions in LiSES.
As part of ongoing efforts to develop LiSES with higher energy density, this paper will report on the characterization of LiSES prepared from corannulene made by the literature procedure9 as well as 1,3,5-triphenylbenzene, TPB synthesized by a slightly modified literature 10. 1,3,5-triphenylbenzene, as shown in Figure 1(1), can be classified as a biphenyl derivative with two additional phenyl rings at positions 3 and 5 of the same ring. Since this molecule has four benzene rings, it should uptake 4 atoms of Li per molecule, which is more than for biphenyl (maximum 2.5 mole equivalents of Li per PAH in 0.5 M solution) and naphthalene (<2.5 mole equivalents of lithium per molecule).
Corannulene is a five-ring PAH arranged into a bowl shape as shown in Figure 1(2). Zabula et al.11 have demonstrated the feasibility of dissolving metallic lithium in a solution of corannulene/tetrahydrofuran (THF) to form a solution with five Li+ ions sandwiched between two stable tetraanions of corannulene.
Figure 1: The molecular structures of 1,3,5-triphenylbenzene (1) and corannulene (2). 1,3,5-triphenylbenzene is classified as a biphenyl derivative with two additional phenyl rings at positions 3 and 5 of the same ring. Corannulene is a five-ring PAH with its five benzene rings arranged into a bowl shape. Please click here to view a larger version of this figure.
Thus, both 1,3,5-triphenylbenzene and corannulene are potential candidates for high energy density LiSES.
Protocol
1. Preparation Procedure for 1,3,5-Triphenylbenzene (1)
- Place a mixture of acetophenone (4.0 g, 33.3 mmol) and 100 ml of absolute ethanol into a round bottom three neck 250 ml flask equipped with magnetic stirrer, reflux condenser, nitrogen inlet, bubbler, dropping funnel and thermometer. Add silicon tetrachloride (11.9 g, 8.0 ml, 70.2 mmol, 2.1 eq.) to the mixture in one portion at 0 °C under nitrogen using the dropping funnel.
- Observe the evolution of gaseous hydrogen chloride for 10 min. Then stir the reaction mixture at 40 °C for 20 hr.
- Cool down the reaction mixture to 23 °C and pour in 200 g of water mixed with ice (1:1 mass ratio).
- Extract the resulting mixture with dichloromethane (2 x 100 ml) using an extraction funnel.
- Wash the combined extracts once with saturated NaCl solution (100 ml), and dry over 15 g of anhydrous MgSO4. Filter the liquid part off and then concentrate using a rotary evaporator.
- Purify the product via recrystallization from ethanol (dissolution in minimal amount of ethanol followed by partial evaporation of the solvent, keeping at 6 °C overnight, and rapid filtration) to obtain 2.2 g (yield 63%) of 1,3,5-triphenylbenzene (1) as pale yellow crystals.
Note: 1H-NMR (400 MHz, CDCl3): δ= 7.41 (m, 3H), 7.50 (m, 6H), 7.72 (d, 6H, J = 7.33Hz), 7.80 (s, 3H). 13C-NMR (400 MHz, CDCl3): δ= 125.21, 127.39, 127.57, 128.88, 141.18, 142.38.
2. LiSES Prepared with 1,3,5-Triphenylbenzene
- Preparation of 1,3,5-triphenylbenzene-based LiSES
NOTE: 1,3,5-triphenylbenzene used in this paper was synthesized as per procedure described above. The 1,3,5-triphenylbenzene -based LiSES are denoted by LixTPB(THF)24.7 where x denotes the Li:PAH molar ratio and TPB denotes 1,3,5-triphenylbenzene. Prepare LixTPB(THF)24.7 inside an argon-filled glovebox at ambient temperature via the following steps:- Measure out well defined quantities of metallic Li, THF and TPB separately inside the glovebox to achieve the target molar composition of LixTPB(THF)24.7 for x = 1, 2, 3, and 4. Use 41.6 mg, 83.3 mg, 124.9 mg, 166.6 mg of Li for x = 1, 2, 3 and 4 respectively.
- For each of the four LiSES samples to be prepared, dissolve 1.84 g of TPB in 12 ml of THF inside four separate glass bottles to form 12 ml of colorless solutions of TPB(THF)24.7 for each bottle. Use a 0.5 M 1,3,5-triphenylbenzene in all the solutions.
- Add the weighed metallic Li foils to the four bottles and seal the bottles with Parafilm.
- Stir the mixture in each bottle overnight using a glass-coated magnetic stirrer to ensure the complete dissolution of metallic Li.
- Conductivity Measurements
- Carry out all conductivity measurements using a standard conductivity cell probe based on the four-electrode technique. Attach the cell probe to a meter. The probe has a secondary function to measure the solution's temperature at the same time and display both conductivity and temperature measurements.
- Prior to measurements, calibrate the meter using 50 ml of standard 0.01 M aqueous KCl solution provided by the conductivity probe's manufacturer outside the glovebox.
- Carry out all the conductivity measurements for 1,3,5-triphenylbenzene-based LiSES, LixTPB(THF)24.7 for x = 1, 2, 3, 4 inside the glovebox.
- For each of these LiSES, pour out the sample into a short glass cylinder and immerse the probe into the solution. Record the conductivity measurement over a period of one to two hours till each sample returns to an ambient temperature. The time taken for each sample to return to ambient temperature is ~1-2 hr. The probe will remain immersed in the sample for the entire duration of conductivity measurement.
3. Corannulene
- Preparation of corannulene-based LiSES
NOTE: The corannulene used in this paper was synthesized at the School of Physical and Mathematical Sciences, NTU using a multistep literature procedure.9 The corannulene-based LiSES are denoted by LixCor(THF)247 where x denotes the Li:PAH molar ratio and Cor denotes the corannulene. Prepare LixCor(THF)247 inside an argon-filled glovebox at ambient temperature via the following steps:- Measure out well defined quantities of metallic Li, THF and Cor separately inside the glovebox to achieve the target molar composition of LixCor(THF)247 for x = 1, 2, 3, 4 and 5. Use 4.2 mg, 8.3 mg, 12.5 mg, 16.6 mg and 20.8 mg of Li for x = 1, 2, 3, 4 and 5 respectively.
- Next, for each of the five LiSES samples (x = 1, 2, 3, 4 and 5) to be prepared, dissolve 0.15 g of Cor in 12 ml of THF inside five separate glass bottles to form 12 ml of colorless solution of Cor(THF)247 in each bottle. Use a corannulene concentration of 0.05 M).
- Next, add the weighed metallic Li foils to the five bottles of Cor(THF)247 and seal the bottles with Parafilm.
- Stir the mixture in each bottle overnight using a glass-coated magnetic stirrer to ensure the complete dissolution of the metallic lithium.
- Conductivity Measurements
- For conductivity versus temperature measurements, remove each of the five bottles containing LixCor(THF)247 for x = 1, 2, 3, 4 and 5 individually from the glovebox, wrap it with an additional layer of para-film and immerse it inside an insulated Styrofoam container filled with dry ice.
NOTE: The LiSES samples did not come into contact with either moisture or oxygen while outside the glovebox because the bottles were sealed. - Cool each bottle down to about 10 °C by keeping each bottle immersed in the dry ice for about 30 min before being transferred back into the glovebox for conductivity measurements.
- Purge the ante-chamber of the glovebox at least 5 times for each cooled sample to ensure that no traces of water of condensation accompany the bottle back into the glovebox.
- Similar to the manner in which conductivity versus temperature measurements were collected for naphthalene-based LiSES samples1, measure the conductivity of LixCor(THF)247 (x = 1, 2, 3, 4, 5) over a period of one to two hours till each sample returned to ambient temperature. The probe will remain immersed in the sample for the entire duration of conductivity measurement.
- For conductivity versus temperature measurements, remove each of the five bottles containing LixCor(THF)247 for x = 1, 2, 3, 4 and 5 individually from the glovebox, wrap it with an additional layer of para-film and immerse it inside an insulated Styrofoam container filled with dry ice.
Representative Results
Reaction between various amounts of lithium and mixtures of 1,3,5-triphenylbenzene with THF gives greenish blue or deep blue colored solutions as shown in Figure 2. A light color indicates that the particular sample of LiSES has a low concentration of solvated electrons. 1,3,5-triphenylbenzene demonstrates increase of conductivity with increase of Li:PAH ratio from 1 to 2 in 0.5 M THF solution (Table 1). However, conductivity value gradually decreases upon further increasing the molar ratio. The conductivity value for Li:PAH = 4 is even lower than for Li:PAH = 1. This behavior is similar to that seen for LiSES made from biphenyl and naphthalene1, 2.
| Mole eq. of Li per 1 | 1 | 2 | 3 | 4 |
| Conductivity (mS/cm) | 1.69 | 2.04 | 1.62 | 1.33 |
Table 1: The conductivity readings (in mS/cm) for Li SES prepared using LixTPB(THF)24.7 (x = 1, 2, 3, 4). LixTPB(THF)24.7 means 0.5 M solution of TPB in THF with different Li mole ratio.

Figure 2: After all the metallic Li had dissolved in TPB(THF)24.7, the colors of LixTPB(THF)24.7 ranged from light blue (for x = 1) to very dark blue (for x = 4). A lighter color indicates a lower concentration of solvated electrons in the TPB(THF)24.7 solution. This photograph shows a solution of Li3TPB(THF)24.7 for x = 3 which has a dark blue color. Please click here to view a larger version of this figure.
For the Cor-based LiSES, when all the metallic Li (for x = 1, 2, 3, 4, 5) had dissolved in Cor(THF)247 , the colors of the LiSES ranged from green (for x = 1) to very dark green (for x = 5). As the concentration of Cor in THF was very low (0.05 M), the volume expansion of the solution versus the amount of Cor dissolved in THF was negligible. The color change of the solution as metallic lithium was dissolved over a period of 24 hr to form Li3.0Cor(THF)247 is shown in Figure 3. The solution's color changed from colorless at t = 0 hr to light green and finally to dark green when all the lithium had dissolved. The temperature dependence of conductivity of LixCor(THF)247 solutions (x = 1, 2, 3, 4 and 5) in the temperature range of 284 K to 298 K is presented in Figure 4. The conductivity versus temperature profiles shows linear trend between σ and T for all five samples with each profile having a negative slope. The data is then used to compute both the conductivity σ0 at T0 and the temperature coefficient α for Table 2.
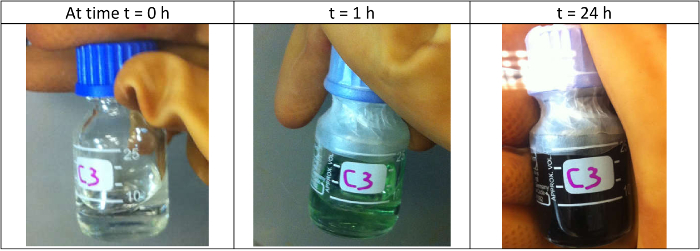
Figure 3: The three photographs in Figure 3 arranged in chronological order show the color change of the solution for Li3.0Cor(THF)247 as metallic Li is dissolved in Cor(THF)247 over 24 hr. The colors range from colorless when the metallic Li is first added (at t = 0 hr) to light green (at t = 1 hr) when some Li has dissolved and finally to dark green (at t = 24 hr) when all the Li is dissolved. Please click here to view a larger version of this figure.
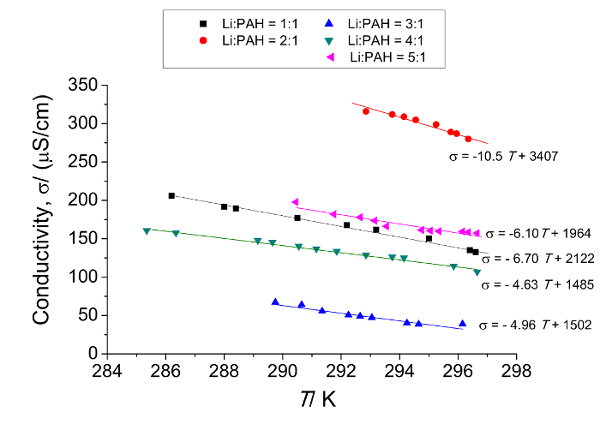
Figure 4: Conductivity versus temperature measurements for LixCor(THF)247 solutions (x = 1, 2, 3, 4 and 5) for the temperature range of 284 K to 298 K, which show linear trends for all 5 samples (x = 1, 2, 3, 4 and 5) with negative gradients. The negative gradients indicate that all these samples exhibit metallic behavior. The conductivity versus temperature data of these 5 samples are used to compute both the conductivity σ0 at T0 and the temperature coefficient α for Table 2. Please click here to view a larger version of this figure.
| x | σ0 (102 µS/cm) | α (10-2 K-1) |
| 1 | 1.25 | 5.36 |
| 2 | 2.77 | 3.79 |
| 3 | 0.23 | 21.7 |
| 4 | 1.04 | 4.44 |
| 5 | 1.45 | 4.20 |
Table 2: σ0 and α for LixCor(THF)247 (for x = 1, 2, 3, 4, 5) based on Equation (1). σ0 and α are both obtained from the conductivity versus temperature data of Figure 4. The results shown in this table indicate an 'x' dependence for both σ0 and α.
Discussion
For the 1,3,5-triphenylbenzene-based LiSES, a sample with a light color shows that it has a low concentration of solvated electrons. LixTPB(THF)24.7 (for x = 1, 2, 3, 4) demonstrates a behavior in its conductivity versus x similar to that seen for LiSES made from biphenyl and naphthalene1, 2.There is an initial increase in conductivity with increase of Li:PAH ratio from 1 to 2 and a subsequent decrease in conductivity upon further increasing the molar ratio to 3 and 4, with conductivity value of Li4TPB(THF)24.7 even lower than for Li1TPB(THF)24.7.
From Figure 4, it can be seen that the relationship between σ and T is linear for all five samples and each profile has a negative slope. This indicates that LixCor(THF)247 exhibits a metallic behavior similar to that of both the biphenyl and the naphthalene-based LiSES1,2. The relationship between σ (µS/cm) and T(K) for LixCor(THF)247 can be expressed as:
σ(x,T) = σ0[1-α(T–T0)] (1)
where σ0 is conductivity at T0 and α is the temperature coefficient and both terms are "x" dependent. The respective data for all five profiles are presented in Table 2.
The low conductivities of all five samples, measured in the range of 102 μS/cm instead of mS/cm can be attributed to the fact that the LixCor(THF)247 solutions are all very dilute in THF as compared to the LiSES that the authors have studied earlier based on biphenyl and naphthalene.
As LiSES are both oxygen and moisture sensitive, the most critical steps in experiments with LiSES are as follows. 1) First, ensure that both processes for preparing LiSES and conductivity measurements are performed completely within the argon-filled glovebox to prevent contact of the LiSES with moisture and oxygen. This is because contact with either moisture or oxygen will lead to the LiSES being neutralized to form hydroxides and oxides of Li which are useless for solvating the electrons and detrimental to the conductivity. 2) Second, ensure that each bottled sample of the LiSES is not in contact with either moisture or oxygen when it is taken out for cooling in dry ice.
A modification of the existing method for stirring solutions is the use of a custom-made borosilicate glass-coated magnetic stirrer for preparing LiSES instead of using Teflon-coated (C2F4)n ones that are readily available in the market. The (C2F4)n reacts upon contact with metallic Li and LiSES to give C and LiF. Visually, the stirrer will have turned black (carbon is left on the stirrer) and the F ions will have gone into the LiSES as LiF and affect the conductivity measurements. As carbon is porous, further use of the now-carbon coated stirrer to stir future LiSES will introduce iron (from the magnet) into the solutions.
The usage of customized glass-coated stirrers for LiSES preparation instead of Teflon-coated stirrers is very significant. Although this may be overlooked as a simple process, black-colored Teflon sticks or Teflon-coated stirrers turning black after use can easily be mistaken as having been made dirty by the stirring process without the realization that 1) LiF is formed with F being stripped from the polymer by the LiSES and mixed into the solution and 2) that the black color actually indicates an irreversible damage of the polymer coating turning into carbon. Hence the existing method of using Teflon coated stirrers does not work for LiSES preparation.
Troubleshooting the technique for cooling LiSES is done to ensure that each of the LiSES sample is not frozen solid during cooling but instead just cooled to about 10 °C in the dry ice. Otherwise, time will be wasted waiting for frozen LiSES to defrost in the glovebox. This is achieved by trial and error in timing (optimum: 30 min) since the bottles cannot be unsealed for temperature measurement of the LiSES outside the glovebox.
There are three limitations for the LiSES experiments. Firstly, as the LiSES are both sensitive to both moisture and oxygen, preparations of the LiSES samples and conductivity measurements must be restricted to the argon environment inside a glovebox. Most conductivity measuring devices available are bulky and cannot fit inside a glovebox. The manufacturers of these devices assume that the user's samples are not air-sensitive. Hence the conductivity measurements described in this paper were made using a handheld meter and probe. Secondly, as described in the protocol section for the cooling experiment, the samples were cooled to about 10 °C before being transferred back inside the glovebox. This temperature is an estimate because the bottles cannot be unsealed outside the glovebox for temperature measurements. Thirdly, the limitation of experimenting with the Cor PAH is that it is very difficult to obtain a large amount of Cor in lab conditions unlike biphenyl or naphthalene. This will rule out the possibility of obtaining a larger quantity to prepare a higher concentration solution of Cor in THF.
The future application of the techniques described here is to study the physical and electrochemical properties of LiSES prepared using other types of PAHs so as to select the ideal candidate as lithium solvated electrons solution anode material for room temperature refillable LiSES batteries.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge funding from the Singapore Ministry of Education Tier 2 Research Fund (project MOE2013-T2-2-002) for this project.
Materials
| Biphenyl Reagentplus, 99.5% | Sigma Aldrich | B34656-1KG | |
| Tetrahydrofuran Anhydrous, 99.9%, Inhibi | Sigma Aldrich | 401757-100ML | |
| Iodine, Anhydrous, Beads, -10 Mesh, 99.999% trace metals basis | Sigma Aldrich | 451045-25G | |
| Lithium iodide, anhydrous, 99.95% (metals basis) | Alfa Aesar | 40666 | |
| Lithium Ion Conducting Glass ceramics (Diameter 1" x 150 mm) | Ohara | LICGC(AG01) | |
| Lithium Foil | Alfa Aesar | 010769.14 | |
| Methanol anhydrous, 99.8% | Sigma Aldrich | 322415-100ML | |
| CompactStat : Electrochemical Analyser with Impedance | Ivium Technologies | Not Applicable | |
| Cond 3310 Conductivity Meter | WTW | Not Applicable | |
| Digital Multimeter, Model Fluke 179 | Fluke Corporation | Not Applicable | |
| 1,3,5-triphenylbenzene | Synthesized from acetpohenone according to procedure described in literature | ||
| Silicon tetrachloride | Sigma Aldrich | 215120-100G | |
| acetophenone | TCI | A0061-500g | |
| Ethanol | Merck Millipore | 1.00983.2511 |
Referencias
- Tan, K. S., Yazami, R. Physical-Chemical and Electrochemical Studies of the Lithium Naphthalenide Anolyte. Electrochim Acta. 180, 629-635 (2015).
- Tan, K. S., Grimsdale, A. C., Yazami, R. Synthesis and Characterisation of Biphenyl-Based Lithium Solvated Electrons Solutions. J Phys Chem B. 116, 9056-9060 (2012).
- Rinaldi, A., Tan, K. S., Wijaya, O., Wang, Y., Yazami, R., Menictas, C., Skyllas-Kazacos, M., Lim, T. M., Hughes, S. Ch. 11. Advances in batteries for large- and medium-scale energy storage applications in power systems and electric vehicles. , (2014).
- Wang, Y., Tan, K. S., Yazami, R. . Materials Challenges In Alternative & Renewable Energy (MCARE 2014). , (2014).
- Yazami, R., Tan, K. S. . in 8th annual Li Battery Power. , (2012).
- Yazami, R. Hybrid Electrochemical Generator With A Soluble Anode. US patent. , (2010).
- Yazami, R., Tan, K. S. Liquid Metal Battery. US patent. , (2015).
- Lim, Z. B., et al. Synthesis and assessment of new cyclopenta-2,4-dienone derivatives for energy storage applications. Synthetic Met. 200, 85-90 (2015).
- Butterfield, A. M., Gilomen, B., Siegel, J. S. Kilogram-Scale Production of Corannulene. Org. Process Res. Dev. 16, 664-676 (2012).
- Elmorsy, S. S., Pelter, A., Smith, K. The direct production of tri- and hexa-substituted benzenes from ketones under mild conditions. Tetrahedron Lett. 32, 4175-4176 (1991).
- Zabula, A. V., Filatov, A. S., Spisak, S. N., Rogachev, A. Y., Petrukhina, M. A. A Main Group Metal Sandwich: Five Lithium Cations Jammed Between Two Corannulene Tetraanion Decks. Science. 333, 1008-1011 (2011).

