An ELISA Based Binding and Competition Method to Rapidly Determine Ligand-receptor Interactions
Summary
The presented protocols describe two enzyme-linked immunosorbent assay (ELISA) based techniques for the rapid investigation of ligand-receptor interactions: The first assay allows the determination of dissociation constant between ligand and receptor. The second assay enables a rapid screening of blocking peptides for ligand-receptor interactions.
Abstract
A comprehensive understanding of signaling pathways requires detailed knowledge regarding ligand-receptor interaction. This article describes two fast and reliable point-by-point protocols of enzyme-linked immunosorbent assays (ELISAs) for the investigation of ligand-receptor interactions: the direct ligand-receptor interaction assay (LRA) and the competition LRA. As a case study, the ELISA based analysis of the interaction between different lambda interferons (IFNLs) and the alpha subunit of their receptor (IL28RA) is presented: the direct LRA is used for the determination of dissociation constants (KD values) between receptor and IFN ligands, and the competition LRA for the determination of the inhibitory capacity of an oligopeptide, which was designed to compete with the IFNLs at their receptor binding site. Analytical steps to estimate KD and half maximal inhibitory concentration (IC50) values are described. Finally, the discussion highlights advantages and disadvantages of the presented method and how the results enable a better molecular understanding of ligand-receptor interactions.
Introduction
A comprehensive understanding of signaling pathways requires detailed knowledge about the ligand-receptor interaction. Most methods for assessing the interaction of a particular ligand with its specific receptor are expensive, time consuming, labor intensive and require specific equipment and expertise 1.
This article describes two fast and reliable point-by-point protocols to investigate the ligand-receptor interaction based on an enzyme linked immunosorbent assay (ELISA): the direct ligand-receptor interaction assay (LRA) and the competition LRA. ELISA is a highly sensitive, specific and readily available technique, routinely used in almost every laboratory. ELISA can be performed and adapted in various fashions. The presented protocols are optimized for the investigation of the interaction between different lambda interferons (INFLs) and their receptor.
The direct LRA allows for a quantification of ligand-receptor binding with respect to ligand concentration and thus yields a binding curve. Using an appropriate model for the ligand-receptor interaction, the data can be further analyzed to estimate the dissociation constant (KD).
In the presented protocol, the commonly used Hill equation is applied to model the ligand-receptor binding. Although other methods such as the surface plasmon resonance technology 2,3 allow the determination of the binding affinities between two proteins, this technology is often labor intensive, expensive, and requires special laboratory equipment.
The competition LRA enables the screening of inhibitory peptides: The ligand-receptor binding is quantified with respect to peptide concentration. This yields a dose-response curve describing the inhibitory effect of the peptide. The data can be further analyzed to estimate the half maximal inhibitory concentration (IC50) of the blocking peptide.
Both ELISA protocols are easy to use and can be adapted to a broad range of research questions. Recombinant proteins of any kind can be used to reliably and fast determine the interaction parts. In addition, the competition LRA can be used to determine critical interaction sites of ligands and receptors by using blocking peptides, which are designed to mimic either the ligand or the receptor. If the blocking peptide shows efficient and specific inhibition, the peptide occupies a critical interaction site of the ligand (if the peptide mimics the receptor) or of the ligand (if the peptide mimics the ligand).
The first protocol describes the KD value determination of different INFLs and the alpha subunit of their receptor, i.e., the interleukin-28 receptor (IL28RA) using the direct LRA. Next, the second protocol shows how to determine the capability of a 20 amino acid long peptide to inhibit the INFL-IL28RA interactions. The peptide is designed to compete with IFNLs at their receptor binding site and thus enables a molecular understanding of the interaction. Furthermore, this peptide can be used to block IL28RA in in vitro experiments to determine the impact on downstream signaling effects4.
Protocol
1. Reagent Preparation
- To prepare carbonate coating buffer, dissolve 0.36 g Na2CO3 and 0.84 g NaHCO3in 100 ml distilled water; sterile filter the buffer by using a vacuum driven 0.22 µm polyethersulfone (PES) membrane filter and store at RT until usage.
- Prepare washing solution by adding 0.05% v/v Tween 20 in Phosphate buffered saline (PBS).
- Prepare a 5% Bovine Serum Albumin (BSA) (blocking solution) in PBS solution by dissolving 5 g BSA (≥98%) in 100 ml PBS and store at 4 °C.
- Recombinant Receptor, Ligands and Blocking Peptides
- Reconstitutethe recombinant human interleukin receptor alpha subunit (IL28RA) and recombinant His-tagged ligands of human IFN (IFNL1-3) according to the manufacturer's instructions and store at -80°C. Synthetize blocking peptides and used as previously described 4. Use PBS to prepare different concentrations of ligands and peptides for use in the assays.
- To prepare the primary antibody, dilute 6x His Mouse monoclonal antibody in PBS with 0.1% BSA at 1:1,000 dilution. To prepare the secondary antibody, dilute horseradish peroxidase (HRP) conjugated goat anti-mouse IgG (H+L) in PBS with 0.1% BSA at 1:10,000 dilution.
- Prepare TMB solution by mixing the reagents A and B according to the manufacturer's instructions.
- Prepare stop solution by adding 5 N sulphuric acid (H2SO4) in distilled water and store at RT.
2. Enzyme-linked Immunosorbent Assays (ELISAs)
NOTE: The direct ligand-receptor interaction ELISA (direct LRA, Figure 1) can be used to measure the receptor-ligand dissociation constant (KD), as a measure of the receptor-ligand binding affinity. The competition ligand-receptor interaction ELISA (competition LRA, Figure 2) allows screening of peptides (and other blocking compounds), which act to interfere with the interaction between ligand and receptor. The basic protocol that was previously published 5 was further optimized.
NOTE: In both ELISA methods use multichannel pipette for adding solutions to the wells of 96-well plate in each step. In solution decant or washing steps, throw out the solutions directly into the sink.
- Direct Ligand-Receptor-Interaction Assay (direct LRA)
NOTE: For an illustration of the workflow (see Figure 1).- Coating Plate with Recombinant Receptor
- Dilute the recombinant receptor in carbonate buffer to a final concentration of 100 ng/µl. Coat wells of 96-well microtitre plate with fixed receptor concentration (100 ng/µl) by pipetting 100 µl to each well using a multichannel pipette. Exclude outer walls of the plate to avoid well edge artifact. Cover the plate with a lid and incubate the plate at 4 °C O/N.
- Blocking and Addition of Ligands
- The next day, remove the coating solution by tilting the plate against the sink and wash the plate 3 times with washing solution (PBS + 0.05% v/v Tween 20).
- Block the free receptor-binding sites in the coated plate using 200 µl of 5% BSA solution to each well using a multichannel pipette and incubate the plate for 2 hr at RT.
- Discard the blocking solution (see step 2.1.2.1.) and wash the plate 3 times with washing solution.
- Prepare the recombinant His-tagged ligands at different concentrations (e.g., 8 µg/ml, 4 µg/ml, 2 µg/ml, 1 µg/ml, 0.5 µg/ml, 0.25 µg/ml, 0.125 µg/ml, 0.063 µg/ml, 0.031 µg/ml, 0.0 µg/ml) in PBS. Add only PBS in the blank wells.
- Add 100 µl of each ligand concentration to the wells in duplicate and incubate the plate for 2 hr at RT allowing receptor-ligand interaction.
- Incubation with Antibodies
- Following incubation with the ligands, wash the plate 3 times with washing solution.
- Pipette 100 µl of primary anti-His mouse monoclonal antibody solution (1:1,000) to each well.
- Incubate the plate at RT for 2 hr; after incubation, discard the antibody solution (see step 2.1.2.1.) and wash the plate 3 times with washing solution.
- Add 100 µl of HRP coupled goat anti-mouse IgG secondary antibody solution (1:10,000) to each well. Incubate the plate for 45 min at RT.
- Discard the antibody solution (see step 2.1.2.1.) and wash the plate 3 times with washing solution.
- Addition of Substrate and Development
- Bring the TMB substrate solutions to RT and prepare TMB substrate solution A and B at 1:1 ratio. Add 100 µl freshly prepared substrate to each well and keep the plate at RT for 15-30 min. After sufficient color development add 50 µl stop solution.
- Reading the Plate and Data Analysis
NOTE: The described protocol is based on the assumption that the measured signal rises from specific binding. It might be necessary to estimate the contribution of unspecific binding to the signal but this is out of the scope of this protocol.- Read the absorbance (optical density, OD) directly at 450 nm.
- Subtract the background signal from the measured OD values and normalize them. Transform all values of the ligand concentration to logarithmic scale (base 10, log10).
- Plot the normalized and background corrected OD values (Y-axis, corresponds to the fraction of occupied receptor binding sites) against the logarithm of the ligand concentration (X-axis, log10 scale).
- To estimate the KD value, fit the data to the following form of the Hill equation:

NOTE: Here Y denotes the fraction of occupied receptor binding sites and Ymax the maximal binding; [L] denotes the concentration of free ligand and the Hill coefficient. If there is only one binding site for the ligand, the Hill coefficient is n = 1. For systems with more than one ligand binding site, the binding exhibits positive cooperativity if n >1, negative cooperativity if n<1 and no cooperativity if n = 1. The microscopic dissociation constant is termed and corresponds to the half maximal effective concentration EC506. The apparent dissociation constant is Kd = (KD)n. In the simplest case where n = 1, the dissociation constant corresponds to the ligand concentration at which half of the receptor binding sites are occupied and Kd = KD. This model assumes mass action binding under equilibrium conditions, as well as that only a small fraction of the added ligand is bound to the receptor, i.e., [L] >> [RL].
- Coating Plate with Recombinant Receptor

Figure 1. Direct ligand-receptor-interaction assay (direct LRA). Step-by-step protocol for direct LRA. Please click here to view a larger version of this figure.
- Competition Ligand-Receptor-Interaction Assay (competition LRA)
NOTE: For an illustration of the workflow see Figure 2. The competition LRA procedure follows the same steps as the direct LRA (coating the plate, antibody incubation, plate development) except for important changes in the ligand and peptides addition step. Proper negative controls are essential for this assay. In a previous screening study 4, the scrambled blocking peptide did not show antagonistic effects.- Blocking – Addition of Ligands and Blocking Peptides
- The next day, remove the coating solution and wash the plate (see 2.1.2.1).
- Block the coated plate by adding 200 µl of 5% BSA solution to each well and incubate the plate for 2 hr at RT.
- Prepare the recombinant His-tagged ligands (IFNL1-3) at a fixed concentration (2x-20 ng/ml) in PBS.
- Prepare the blocking peptide (cf. Table 3) with different concentrations ranging from 10 nM to 100 µM in PBS to guarantee a dose-response curve.
NOTE: This enables subsequent determination of the IC50 value for the blocking peptide. In control wells, add only fixed ligand concentration without peptide to derive the maximum (100%) binding. In the blank, add only PBS without ligand or peptide. - Add 50 µl of the ligands (IFNL1-3) and 50 µl of each peptide concentration to the wells in duplicates.
- Incubate the plate for 2 hr at RT.
- Reading the Plate and data Analysis
NOTE: The described protocol is based on the assumption that the measured signal rises from specific binding. It might be necessary to estimate the contribution of unspecific binding to the signal but this is out of the scope of this protocol.- Read the absorbance (optical density, OD) directly at 450 nm.
- Subtract the background signal from the measured OD values and normalize them. Transform all values of the peptide concentration to logarithmic scale (base 10, log10).
- Plot the normalized and background corrected OD values (Y-axis, corresponds to the fraction of occupied receptor binding sites) against the logarithm of the ligand concentration (X-axis, log10 scale).
- To estimate the IC50 value, fit the data to the following equation:

NOTE: Here [P] is the peptide concentration and the Hill slope. The Hill slope describes the steepness of the dose-response curve. The IC50 corresponds to the inhibitor concentration at which 50% inhibition of binding between ligand and receptor is observed.
- Blocking – Addition of Ligands and Blocking Peptides
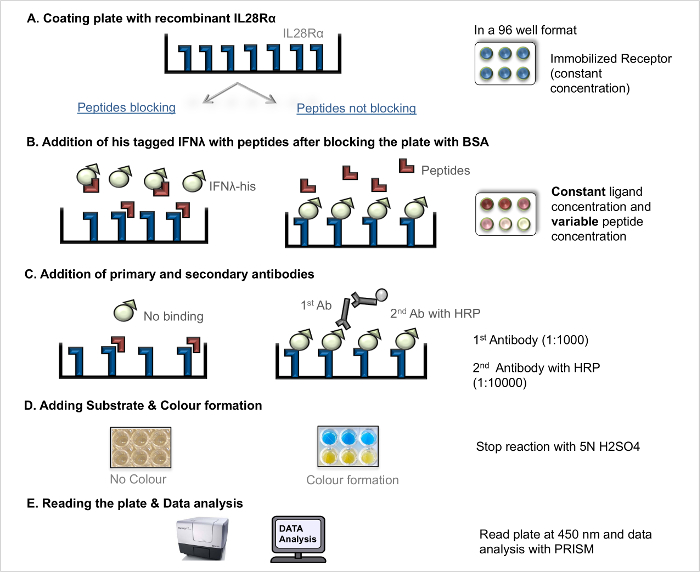
Figure 2. Competition ligand-receptor-interaction assay (competition LRA). Step-by-step protocol for competition LRA. Please click here to view a larger version of this figure.
Representative Results
The dissociation constants between INFL1-3 and their receptor alpha subunit IL28RA were determined using the direct LRA. The results are shown in Figure 3: The fraction of occupied binding sites is plotted against the logarithm of the respective IFN concentration. The Scatchard plot of the data is shown in the bottom right corner. The results illustrate that the direct LRA yields a binding curve, which can be further analyzed to estimate the KD value. The KD value was determined by fitting the data to the Hill equation (Equation 1).
IFNL1 has the highest binding affinity, followed by IFNL2 and IFNL3. The Hill coefficient n >1 suggests increased affinity for additional ligands after the initial ligand-receptor interaction (see Discussion). The estimated dissociation constants and Hill coefficients are summarized in Table 1.
Competitive LRA was used to quantitate the impact of a blocking peptide on the interaction between IFNL1-3 and the IL28RA (Figure 4). The fraction of occupied binding sites for a ligand concentration of 10 ng/ml is plotted against the logarithm of the peptide concentration. To estimate the IC50 values, the data is fitted to Equation 2.
The blocking peptide inhibited the interaction between IFNL3 and IL28RA (IC50 = 0.26 μM) to the greatest extent. The IC50 is twice as high for the IFNL2-IL28RA interaction (IC50 = 0.50 μM) and one order of magnitude higher for the IFNL1-IL28RA interaction, indicative the peptide was less effective at disrupting IFNL1-IL28RA interactions. The determined IC50 values and Hill slopes are summarized in Table 2.
Proper data analysis is essential for understanding the ligand-receptor interaction. The shown results were generated using a scientific graphing software such as GraphPad PRISM. For the KD value determination, the data was fitted to the 'One site – specific binding with Hill slope' (corresponds to Equation 1 in Sec. 2.2.1). For the IC50 value determination, the data is fitted to the function 'log(inhibitor) vs. normalized response – variable slope' (see Equation 2 in Sec. 2.2.2). However, any software for non-linear regression analysis can be used.

Figure 3. Results of the direct ligand-receptor assay (direct LRA). Binding curves for the binding of IFNL1 (green), IFNL2 (red) and IFNL3 (blue) to IL28RA. The respective Scatchard plot in the bottom right corner suggests positive co-operativity of the binding. Please click here to view a larger version of this figure.

Figure 4. Results of the competitive ligand-receptor assay (competitive LRA). Dose-response curves showing inhibition of the binding of IFNL1 (10 ng/ml, green), IFNL2 (10 ng/ml, red) and IFNL3 (10 ng/ml, blue) to IL28RA by the 20 aa peptide. Please click here to view a larger version of this figure.

Table 1: Estimated dissociation constants (KD) and Hill coefficients of IFNL1-3 binding to IL28RA. The standard error (SE) is given for a sample size of four replicates per data point.

Table 2: Estimated half maximal inhibitory concentrations (IC50) of the blocking peptides and the Hill slope of the dose-response curve for the binding of IFNL1-3 to IL28RA. The IFN concentration is 10 ng/ml. The number of replicates is three.
Discussion
ELISA is a standard and well-established method for many laboratories. We have further modified and improved a previously published method 5,7. The demonstrated step-by-step protocol shows how it can be used in a simple way to determine the KD values of ligand-receptor interactions. In addition, the IC50 of a blocking peptide that interferes with the ligand-receptor interaction can be determined.
Major advantages are the rapid setup, easy preparation of reagents and familiar handling, as most researchers have used an ELISA protocol before. The direct LRA protocol is highly flexible and can be adapted to measure many protein-protein interactions. Recombinant proteins with His6- or alternative tag should be used as the binding partner to an immobilized partner. The competition LRA can be exploited as a screening tool to (i) determine the inhibitory potential of blocking compounds (peptides, antibodies, or small molecules) and to (ii) determine the critical interaction sites by using blocking peptides designed to mimic the receptor or the ligand.
Negative controls are essential for a proper interpretation of the presented assays. In a previous screening study 4, the scrambled sequence of the used blocking peptide did not show antagonistic effects. However, other peptides showed a blocking capacity also after scrambling, likely due to unspecific electrostatic interactions.
A potential limitation is that this assay reflects an in vitro situation. In particular, heterodimeric receptors often form a more complex structure. It is not possible to distinguish whether the ligand just binds to the receptor or whether the ligand also activates the receptor by triggering a conformational change or a dimerization, which in turn leads to an intracellular signal. In the presented assay, we used a recombinant receptor, which is immobilized to a solid phase. This setup does not work to test the activation or to investigate the interaction of receptors, which require the membrane environment or membrane cholesterol such as G protein coupled receptors (GPCRs). Also the use of recombinant protein raises caveats. For example, the folding and tertiary structure of a recombinant protein may be different compared to an in vivo situation. The binding of ligand and receptor usually occurs at RT, however in humans the optimal temperature would be 37 °C. Finally, the use of commercial recombinant ligand and receptor can prove expensive. Despite these limitations, these two ELISA protocols show potential to rapidly explore the ligand-receptor interaction.
The presented results show that IFNL1 has a slightly higher affinity for IL28RA compared to IFNL2, and the affinity of IFNL3 is three-fold lower than IFNL2. This is remarkable considering the similarity between IFNLs. IFNL1 and IFNL2 differ in 33 amino acids while IFNL2 and IFNL3 differ only in seven amino acids 8. The interaction between IL28RA and IFNLs involves Helix A and the AB-loop of IFNL 9. Alignment of IFNL sequences reveals four significant differences in Helix A and the AB-loop between IFNL1 and IFNL3 (Figure 5A). One affects the salt bridge Arg54-Glu119. The amino acid residues in this section are enumerated according to the UniProt entries Q8IU54 (IFNL1), Q8IZI9 (IFNL3), Q8IZJ0 (IFNL2) and Q8IU57 (IL28RA), which has been found in the crystal structure of the IFNL1-IL28R1 complex (Figure 5B). Structural alignment shows that Arg57 in IFNL1 is replaced by Lys57 in INFL3, which is also able to form a salt bridge with Glu118 (Figure 5C). Consistent with the decreased affinity of IFNL3 and IL28RA, computational 10,11 and mutational 12 analyses show, that Lys-Glu salt bridges are in general less stable than Arg-Glu salt bridges.
However, the differences in Helix A do not satisfactorily explain the lower affinity of IFNL3, since the amino acid sequence of Helix A is identical for IFNL2 and IFNL3. It is thought that the main difference arises from the mutations in the AB-loop, where Arg74 and His76 in IFNL2 are replaced by Lys70 and Arg72 in IFNL3 8.
Moreover, differences in stability and solubility between IFNLs may also affect the outcome of the assay. Since the direct LRA assay uses concentrations from nM to M and the physiological concentration of cytokines in serum lies in the pM to nM range, aggregation of IFNL cannot be excluded. We can assume that the observed positive cooperativity of the IFN binding is caused by a specific or non-specific increase in ligand-receptor binding, e.g., a dimerization of the recombinant IL28RA receptor in solution, or a binding of a second ligand or ligand fragment with higher affinity. However, further studies are required to verify this.
The blocking peptide used in the competition LRA mimics the AB-loop of IFNL3 (Figure 6). As previously described, the AB-loop plays an essential role in the interaction between IFNLs and IL28RA 9, particularly IFNL2 and IFNL3. Homology modeling of IFNL3 with the IL28RA/IFNL1 crystal structure shows that the region, which corresponds to the blocking peptide lies in close spatial proximity to the interaction interface with IL28RA (Figure 6). Supposed that the peptide blocks the interaction of the AB-loop of IFNL and IL28RA. The results of the competition LRA support this: The peptide has an inhibitory effect on the binding of all IFNLs, however the peptide is a more effective inhibitor of the interaction between IL28RA and either IFNL3 or IFNL2 than of the interaction of IFNL1 and IL28A. As expected, the peptide blocks the binding of IFNL3 most effectively since it occupies exactly the same binding pocket as the AB-loop of IFNL3.
As shown in Table 3, the AB-loops of IFNL1 and IFNL2 aligned to the blocking peptide differ. Consistent with the lower IC50 value of the peptide for inhibition of the IFNL1/IL28RA interaction, twelve amino acids differ between IFNL1 and the peptide whereas and only two amino acids differ between IFNL2 and the peptide. This indicates that there are slightly different binding modes for interaction between the AB-loops of the IFNLs and IL28RA and predicts that peptides which mimic IFNL1 would be more effective at blocking the interaction between IFNl1 and IL28RA than the interaction between IFNL3 and IL28RA.
Further, the peptide is overall positively charged (four arginine and two lysine residues but only two aspartate residues) and computational analysis shows that the peptide has no defined secondary motifs. Due to its coiled, flexible structure the peptide might also bind to other regions of the receptor. This could potentially block glutamate and aspartate residues such as D118, which forms a salt bridge to stabilize the ligand-receptor complex (Figure 5B and 5C).

Figure 5. Structural comparison of IFNL1 (green) and IFNL3 (blue). Oxygen atoms are shown in red, nitrogen atoms are shown in blue. (A) Superposition of the IL28RA-bound IFNL1 and IFNL3 (IL28RA not shown). The root mean square deviation (RMSD) of all aligned atoms is 0.672 Å. Side chains of IFNL3 that differ from IFNL1 are highlighted in light-pink. (B) Salt bridge between Arg54 and D118. (C) IFNL3 aligned to IL28RA-bound IFNL1 (IL28RA is shown in grey). The alignment shows that the Arg-Glu salt bridge is probably replaced by a less stable Lys-Glu, a salt-bridge between Lys57 and D118. PyMol was used for the preparation of figures and for the alignment. Please click here to view a larger version of this figure.

Figure 6. Alignment of IFNL3 and the IFNL1-IL28RA complex (IFNL1 not shown). IFNL3 is shown in blue and the IL28RA in grey. The regions corresponding to the blocking peptide are highlighted in purple. Please click here to view a larger version of this figure.

Table 3: Sequence alignment of IFNL1-3 and inhibitory peptide. The peptide mimics the AB-loop of INFL3. Amino acid mismatches are highlighted in red.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We thank Prof. J. Stelling (Department of Biosystems Science and Engineering, ETH Zurich and Swiss Institute for Bioinformatics, Basel, Switzerland) for his critical review of the manuscript.
Materials
| Nunc-Immunoplate (F96 Maxi sorp) | Thermo Scientific | 442404 | ELISA plate |
| Sodium carbonate (Na2CO3) | Merck | 497-19-8 | For ELISA plate coating buffer |
| Sodium hydrogen carbomnate(NaHCO3) | Merck | 144-55-8 | For ELISA plate coating buffer |
| Bovine Serum Albumin (BSA) | Sigma | A7030-100G | 5% BSA in PBS for Blocking |
| rhIL-28Rα/IFNλR1 | R&D systems | 5260-MR | Recombinant human interlukin-28 Receptor alpha |
| rhIL-29/IFNλ1 | R&D systems | 1598-IL/CF | Recombinant human interlukin-29/Carrier free/C-terminal 10-His tag |
| rhIL-28A/IFNλ2 | R&D systems | 1587-IL/CF | Recombinant human interlukin-28A/Carrier free/C-terminal 6-His tag |
| rhIL-28B/IFNλ3 | R&D systems | 5259-IL/CF | Recombinant human interlukin-28B/Carrier free/C-terminal 6-His tag |
| 6X His Monoclonal antibody (Mouse) | Clontech | 631212 | Primary antiboy to capture His tagged Ligands |
| Goat anti-Mouse igG (H+L) | Jackson Immuno Research | 115-035-166 | Horseradish Peroxidase conjucated secondary antibody |
| BDoptEIA TMB reagent set | BD Biosciences | 555214 | ELISA – TMB substrate solution |
| Sulfuric acid (H2SO4) | Fulka | 84720 | 5N H2SO4 (Enzyme reaction stop solution) |
| Synergy/H1 – Microplate reader | BioTeK | ELISA plate reader |
Referencias
- Schneider, P., Willen, L., Smulski, C. R. Tools and techniques to study ligand-receptor interactions and receptor activation by TNF superfamily members. Methods in enzymology. 545, 103-125 (2014).
- Rossi, G., et al. Biosensor analysis of anti-citrullinated protein/peptide antibody affinity. Analytical biochemistry. 465, 96-101 (2014).
- van der Merwe, P. A., Barclay, A. N. Analysis of cell-adhesion molecule interactions using surface plasmon resonance. Curr Opin Immunol. 8, 257-261 (1996).
- Egli, A., et al. IL-28B is a key regulator of B- and T-cell vaccine responses against influenza. PLoS Pathog. 10, e1004556 (2014).
- Rosenbluh, J., et al. Positively charged peptides can interact with each other, as revealed by solid phase binding assays. Analytical biochemistry. 352, 157-168 (2006).
- Goutelle, S., et al. The Hill equation: a review of its capabilities in pharmacological modelling. Fundamental & clinical pharmacology. 22, 633-648 (2008).
- Levin, A., et al. Peptides derived from HIV-1 integrase that bind Rev stimulate viral genome integration. PLoS One. 4, e4155 (2009).
- Egli, A., Santer, M. D., O’Shea, D., Tyrrell, D. L., Houghton, M. The impact of the interferon-lambda family on the innate and adaptive immune response to viral infections. Emerging infectious diseases. , e51 (2014).
- Gad, H. H., Hamming, O. J., Hartmann, R. The structure of human interferon lambda and what it has taught us. J Interferon Cytokine Res. 30, 565-571 (2010).
- Folch, B., Rooman, M., Dehouck, Y. Thermostability of salt bridges versus hydrophobic interactions in proteins probed by statistical potentials. Journal of chemical information and modeling. 48, 119-127 (2008).
- Yuzlenko, O., Lazaridis, T. Interactions between ionizable amino acid side chains at a lipid bilayer-water interface. The journal of physical chemistry. B. 115, 13674-13684 (2011).
- Tissot, A. C., Vuilleumier, S., Fersht, A. R. Importance of two buried salt bridges in the stability and folding pathway of barnase. Bioquímica. 35, 6786-6794 (1996).

