Subcutaneous Administration of Muscarinic Antagonists and Triple-Immunostaining of the Levator Auris Longus Muscle in Mice
Summary
We describe procedures for repeated administration of inhibitors of muscarinic signaling to the levator auris longus (LAL) muscle of young adult mice and for subsequent immunostaining of its neuromuscular junctions (NMJs) in wholemounts. The LAL muscle has unique advantages for revealing in vivo pharmacological effects on NMJs.
Abstract
Hind limb muscles of rodents, such as gastrocnemius and tibialis anterior, are frequently used for in vivo pharmacological studies of the signals essential for the formation and maintenance of mammalian NMJs. However, drug penetration into these muscles after subcutaneous or intramuscular administration is often incomplete or uneven and many NMJs can remain unaffected. Although systemic administration with devices such as mini-pumps can improve the spatiotemporal effects, the invasive nature of this approach can cause confounding inflammatory responses and/or direct muscle damage. Moreover, complete analysis of the NMJs in a hind limb muscle is challenging because it requires time-consuming serial sectioning and extensive immunostaining.
The mouse LAL is a thin, flat sheet of muscle located superficially on the dorsum of the neck. It is a fast-twitch muscle that functions to move the pinna. It contains rostral and caudal portions that originate from the midline of the cranium and extend laterally to the cartilaginous portion of each pinna. The muscle is supplied by a branch of the facial nerve that projects caudally as it exits the stylomastoid foramen. We and others have found LAL to be a convenient preparation that offers advantages for the investigation of both short and long-term in vivo effects of drugs on NMJs and muscles. First, its superficial location facilitates multiple local applications of drugs under light anesthesia. Second, its thinness (2-3 layers of muscle fibers) permits visualization and analysis of almost all the NMJs within the muscle. Third, the ease of dissecting it with its nerve intact together with the pattern of its innervation permits supplementary electrophysiological analysis in vitro9,5. Last, and perhaps most importantly, a small applied volume (˜50μl) easily covers the entire muscle surface, provides a uniform and prolonged exposure of all its NMJs to the drug and eliminates the need for a systemic approach1,8.
Protocol
1. Subcutaneous administration of muscarinic acetylcholine receptor (mAChR) antagonists
- Prepare under aseptic conditions the appropriate dose of mAChR antagonist, (cf., Table) by dissolving the drug in sterile physiological saline in 1.5mL reaction tube. The following antagonists were used: atropine, Methoctramine, 4-DAMP, AFDX-116, AFDX-384, MT 7.
- Draw 50μl of solution into a1cc insulin syringe and use a separate syringe for each mouse. Also prepare syringes containing physiological saline only for each mouse in the control group. Keep syringes on ice until ready to inject.
- Anesthetize mice with ketamine-xylazine cocktail (120 mg/kg ketamine, 8 mg/kg xylazine) Check for appropriate anesthetic depth by observing lack of response to toe pinch.
- After proper sedation, shave hair covering the rostral region of the head to the caudal region of the neck, wipe remaining hair away with 70% ethanol.
- Place mouse under stereomicroscope, and insert needle parallel to LAL muscle while pulling up lightly on the needle of the syringe (Fig. 1A,B). This ensures that the needle is inserted into overlying connective tissue and does not directly damage the LAL muscle itself. Insert the needle so that the tip covers the caudal aspect of the LAL muscle.
- Very slowly begin to inject solution over the right LAL muscle while withdrawing the syringe; the total injection time should be approximately 1 minute. If injected properly, the solution should form a “dome” that is roughly 1 cm in diameter, in the overlying skin that will remain for at least one hour. A uniformly injected solution will cover the entire rostral and caudal regions of the right LAL muscle.
- Repeat the procedure every 12 hours for 1 to 7 days. For other studies1, we used the same protocol to inject growth factors every 12 hours for up to 21 days. No abnormal responses were observed from mice administered with anesthesia 2x a day for up to 21 days.
2. Dissection of LAL muscles
- Euthanize mice with an overdose of sodium pentobarbital ( 390 mg/kg). Death is assured by assessing lack of heartbeat after thoracotomy is performed.
- Pin down the mouse in a dissection dish, dorsal side up with 1 pin in each paw and 1 pin through the nose.
- Make the first incision through the skin only, using small spring scissors to cut along the left LAL from the region just proximal to the ear to the region around the left shoulder blade.
- Carefully remove the skin in this region, but avoid cutting too close to the right ear as this is one of the points of attachment for the right LAL muscle.
- Locate the centrally positioned adipose tissue strip of lipid along the midline, on the superficial surface of the head, where the right and left LAL muscles meet. Using small spring scissors, cut 1cm to the right of the fat tissue (toward the left ear ) from the proximal edge of the left LAL muscle until reaching the shoulder (Fig. 1C).
- Once the incision is made, peel back the right LAL muscle with small forceps to expose the ventral side of the muscle. Trim the connective tissue and fascia while carefully pulling up on the right LAL muscle with forceps (Fig. 1C).
- Cut around the caudal end of the right LAL at the base of the ear. Continue cutting, moving towards the rostral end of the right ear, keeping the right LAL turned over (Fig. 1C).. It is better to include more tissue at this step than risk damaging the LAL itself. If the muscle begins to dry out, pipette 1X PBS over the muscle.
- Place dissected right LAL in 30mm culture Sylgard dish containing 1X PBS, dorsal side up. Pin down four corners of muscle with small insect pins.
- Using small forceps and spring scissors, clean connective tissue from dorsal and ventral surfaces of the right LAL muscle. Turn muscle over and re-pin in order to clean off ventral surface. 2-3 muscles may be placed in the same 30mm dish.
3. Triple immunostaining of LAL NMJs: Day 1
- Before beginning immunostaining, prepare all needed reagents. 1X PBS, 2% bovine serum albumin BSA)/PBS, 0.1M Glycine in 2%BSA/PBS, 0.2% Triton X100 in 2% BSA/PBS, and 4% paraformaldehyde (PFA) in 1X PBS will be needed. Pre-cool methanol in -20°C freezer.
- Leave LAL muscles pinned in the dish dorsal side up during this process. Discard 1X PBS solution and add enough 4% (PFA) to cover the muscle. Place dish on shaker set at speed 2 or 3 for 20 minutes. Unless otherwise noted, the dish should be placed on a shaker for the following steps.
- Discard 4% PFA and wash the muscle with 1X PBS 3 times, 10′ each.
- Discard last 1X PBS wash and add 0.1M Glycine in 2% BSA/PBS solution for 30′.
- Discard 0.1M Glycine solution and add 1X PBS containing rhodamine conjugated α-bungarotoxin at a concentration of 1:200 for 15′.
- Discard α-bungarotoxin solution and wash muscle in 1X PBS, 3 times 10′ each.
- Permeabilize tissue by adding pre-cooled methanol and place the dish in the -20°C freezer for precisely 5 minutes, no shaking needed.
- Remove methanol and wash 3 times 10′ each with 1X PBS. Discard last wash and block tissue with 0.2% triton X-100 in 2% BSA/PBS for 1 hour. The washing and blocking steps are performed at room temperature.
- Incubate the muscles overnight, shaking, at 4°C in a cocktail of primary antibodies diluted in the blocking solution (cf., Table).
4. Triple immunostaining of LAL NMJs: Day 2
- Wash muscles in 1X PBS 3 times, 10′ each at room temperature.
- Add cocktail of secondary antibodies in blocking solution for 1 hour at room temperature (cf., Table). The muscles must be protected from light from this step onward to prevent photo-bleaching of Alexa-Fluor 647 fluorescence.
- Wash muscles in 1X PBS 3 times, 10′ each.
- Clean off any remaining connective tissue in 1X PBS, cut the lateral borders of the LAL muscles and mount on slides dorsal side up, using glass cover-slips and mounting media. Use clear nail polish to secure cover slip in place.
- Protect slides from light and store at -20°C until ready for analysis.
5. Confocal imaging of LAL NMJs
- High-resolution confocal images are obtained with a Leica Plan Apo 63X oil objective (1.4NA) on a Leica TCS 4D confocal microscope.
- Fluorescein and Alexa 647 signals were simultaneously scanned with laser lines 488 nm (Argon, 488 nm, 20 mW) and 633 nm (HeNe, 633 nm, 10 mW), respectively. Sequentially, Rhodamine signal is then scanned with the laser line 561 nm (DPSS, 561, 20 mW). Optical sections were collected at 0.3 μm intervals, and the number of images in the z-plane varied from 60-80. The pinhole is adjusted to Airy 1 (107.97 um). The image format 1024 x 1024 (X,Y), with a zoom factor of 1, at 400Hz speed results in an image size of 234.32 μm x 234.32 μm and pixel size of 229.05 nm x 229.05 nm.
- Leica TCS-NT acquisition software is then used to reconstruct z-series images into maximum intensity projections.
- Multipanel images are adjusted for brightness and contrast using Adobe Photoshop.
- For a typical LAL muscle, one should be able to analyze approximately 75-100 en face NMJs. Synaptic stability is determined by features such as spatial alignment of pre-, peri- and postsynaptic elements (i.e., nerve, Schwann cells and muscle), and measurements of synaptic area (i.e., synapse size).
Representative results:
At the adult mammalian NMJ, a single motor axon elaborates fine branches that form highly differentiated arbors of a nerve terminal (Fig. 2; Green) on a single muscle fiber, precisely apposed to postsynaptic clusters of nicotinic AChRs (Fig. 2; Red). Perisynaptically, terminal Schwann cells tightly cover all the branches of presynaptic nerve terminals (Fig. 2; Blue). The structural and functional integrity of this tripartite organization is severely perturbed by daily application of subtype-specific mAChR inhibitors. In the example presented here (Fig. 3), 4-DAMP, a mAChR antagonist with high affinity for M1, M3, M4 and M5 mAChR subtypes , evokes selective elimination of nerve terminals from numerous NMJs throughout the muscle surface (Fig. 3B, C). In addition, terminal Schwann cells are abnormally quiescent8 as evidenced by bright S100 labeling without process extension (Fig. 3B”, 3C”). Postsynaptically, muscle fibers are normal and there is no loss of nAChRs (Fig. 3B’, 3C’).
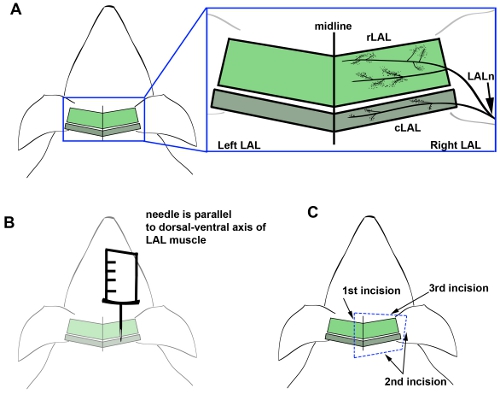
Figure 1. Anatomical location and organization of the LAL muscle. Location of rostral (rLAL), caudal (cLAL), and the LAL nerve (LALn) and corresponding endplate bands are shown (A, inset). The location and orientation of the needle in respect to the LAL muscle is shown for the injection procedure (B). Incision points are shown for the dissection procedure (C).
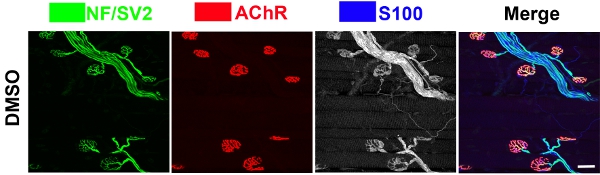
Figure 2. Tripartite organization of the NMJ. High-magnification confocal views of a mouse NMJ. A single axon elaborates fine terminal branches (A), tightly covered by terminal Schwann cell and their processes (A’, asterisks point to terminal Schwann cell bodies). Postsynaptically in a muscle fiber, a cluster of nicotinic AChRs is precisely apposed to the branches of nerve terminals and terminal Schwann cells.
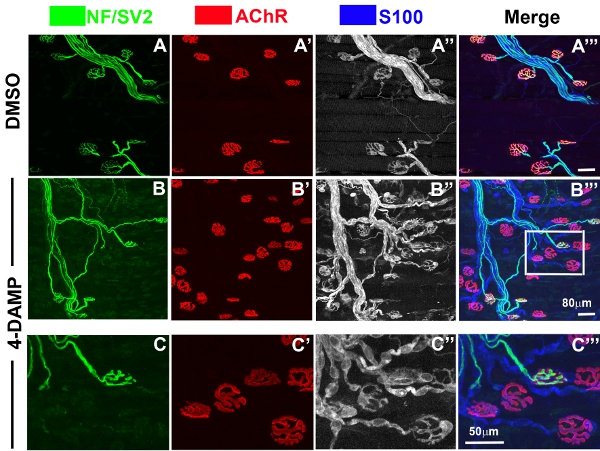
Figure 3. LAL muscles treated with 4-DAMP, a mAChR antagonist. Low and high-magnification confocal views of LAL muscles treated with vehicle or 4-DAMP. In contrast to the vehicle-treated muscle (A-A”’), numerous NMJs in the 4-DAMP-treated muscle lack nerve terminals (B-B”’, C-C”’). A boxed area in Figure 2B is zoomed in Figure 2C.
Discussion
The method presented here permits investigation of previously unrecognized roles of subtype-specific mAChR signaling in the stability and maintenance of mammalian NMJs. This method will also be useful to test the effects of neurotrophic factors and pharmacological agents. For example, our laboratory found that Ciliary Neurotrophic Factor (CNTF) elicited sprouting from nearly all LAL nerve terminals in adult mice1. This result contrasted with prior studies of CNTF-treated hind limb muscles, which reported moderate sprouting at ca. 13-33% of gluteus and at 9% of lateral gastrocnemius junctions3. We believe the discrepancy was due to more uniform and prolonged exposure of nerve terminals to CNTF in LAL than in hind limb muscles. Indeed, when we applied CNTF to lateral gastrocnemius and tibialis anterior muscles using the same protocol that elicited widespread sprouting from nearly all LAL NMJs, we observed weak sprouting from only a modest number of NMJs that was preferentially located near the injection sites. Apparently, exposure of the hindlimb NMJs to CNTF had been limited and uneven, as also noted in a previous study2. On the other hand, CNTF injected between the subdermal connective tissue and the LAL fascia, but not CNTF injected subcutaneously into hind limb muscles, formed a local, subdermal swelling that persisted for at least one hour before vascular reabsorption. It is also notable that even when the injection frequency of CNFT or mAChR antagonists was increased to up to four times daily, we did not observe particularly additive effects of CNTF and mAChR antagonists. In addition, if the injection procedure is performed appropriately, it is unlikely that any direct muscle damage would occur. However, if one did damage the muscle during the injection procedure, axonal sprouting at the nerve terminal or at the nodes, and/or muscle fiber degeneration could be observed1,8. In summary, LAL muscles are a unique preparation that permits uniform, prolonged exposure of all NMJs within a muscle with relatively easy and quick procedures.
The narrower caudal portion of a LAL muscle is thicker (3-5 muscle fibers thick) than the wider rostral portion (2-3 muscle fiber thick). Accordingly, NMJs associated with muscle fibers deeply positioned in caudal LAL are often either not labeled in wholemount immunostaining or labeled only by α-bungarotoxin, which penetrates more deeply than antibodies due to its smaller size and high affinity for nAChRs. These NMJs can be misinterpreted as having lost nerve terminals or Schwann cells due to specific effects of the applied drugs. It is therefore important to note whether these junctions are only observed in caudal LAL, and whether the NMJs or muscle fibers are superficially or deeply positioned: superficially positioned muscle fibers are usually associated with much higher background immunofluorescence than deeply positioned fibers. Alternatively, one can omit deeply positioned NMJs in the caudal strip of the LAL from wholemount analysis.
Although complete transection of LAL nerves is relatively easy and permitted us to study the effects of mAChR inhibition on denervated muscles, the size and accessibility of the LAL nerves limit the types of surgical approaches that can be investigated. For example, partially damaging LAL nerve in order to induce partial denervation of a LAL muscle seems technically quite challenging.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by Muscular Dystrophy Association, NIH (NS062320).
Materials
| Name of the reagent | Company | Catalogue number | Comments |
|---|---|---|---|
| ketamine | Hospira | NDC0409-2051-05 | Dose: 120mg/kg |
| xylazine | Lloyd Laboratories | LA33806 | Dose: 8mg/kg |
| atropine | Sigma-Aldrich | A0132 | (>98% purity); Dose: 0.2mg/kg – 20mg/kg |
| atropine | Voigt Global Distribution | AT105 | Pharmaceutical grade |
| Methoctramine | Sigma-Aldrich | M105 | Dose: 100 – 400M |
| 4-DAMP | Sigma-Aldrich | D142 | Dose: 2.5mg/kg |
| AFDX-116 | Tocris Bioscience | 1105 | 250M |
| AFDX-384 | Tocris Bioscience | 1345 | 50M – 500M |
| MT 7 | Peptides International | PMT-4340-s | 0.1M – 1M |
| 1X Phosphate Buffered Saline, pH 7.4 | Invitrogen | 10010049 | |
| Paraformaldehyde | Fisher | T353-500 | Make 10% solution first by dissolving 10g/100mL de-ionized distilled water; make 4% with 1X PBS, adjust pH to 7.4 |
| Sodium pentobarbitol | Virbac Animal Health | NDC-051311-050-01 | Dose: 390mg/kg |
| Sylgard | Dow Corning | Part # 184 | Follow instructions that come with kit, can use multiple sized culture dish (30mm, 60mm, 100mm) depending on needs |
| 0.1M Glycine | Sigma-Aldrich | G-7126 | Add 0.185g to 25mL of 2% BSA/PBS |
| 2% Bovine serum albumin (2% BSA) | Sigma-Aldrich | A3059-100g | Dissolve 2g BSA into 100mL of 1X PBS |
| 0.2% Triton X100 in 2% BSA/PBS (Blocking Buffer) | Sigma-Aldrich | T9284-100mL | Dissolve 0.2ml/100mL 2% BSA/PBS |
| α-bungarotoxin | Invitrogen | T1175 | Use at concentration of 1:200 |
| SMI-312 | Sternberger Monoclonals | SMI312 | Use at concentration of 1:1000 |
| SV2 | Developmental Studies Hybridoma Bank | SV2-Supernatant | Use at concentration of 1:10 |
| S100 | Dako | Z0311 | Use at concentration of 1:400 |
| FITC- goat anti-mouse IgG1 | Roche | 03117731001 | Use at concentration of 1:200, but if background is high, try 1:400 |
| Alexa-Fluor 647 conjugated goat anti-rabbit | Invitrogen | A21244 | Use at concentration of 1:200 |
| Vectashield fluorescent mounting media | Vector laboratories | H-1000 | This is not a hard-set media, you will need to secure the cover slip with clear nail polish. |
| Small Spring Scissors | Fine Science Tools | 15002-08 | |
| Dissection forceps | Fine Science Tools | 11295-51 |
Referencias
- Wright, M. C., Son, Y. J. Ciliary neurotrophic factor is not required for terminal sprouting and compensatory reinnervation of neuromuscular synapses: re-evaluation of CNTF null mice. Exp Neurol. 205, 437-448 (2007).
- Gurney, M. E., Yamamoto, H., Kwon, Y. Induction of motor neuron sprouting in vivo by ciliary neurotrophic factor and basic fibroblast growth factor. J Neurosci. 12, 3241-3247 (1992).
- Caroni, P., Aigner, L., Schneider, C. Intrinsic neuronal determinants locally regulate extrasynaptic and synaptic growth at the adult neuromuscular junction. J Cell Biol. 136, 679-692 (1997).
- Witzemann, V., Brenner, H. R., Sakmann, B. Neural factors regulate AChR subunit mRNAs at rat neuromuscular synapses. J Cell Biol. 114, 125-141 (1991).
- Angaut-Petit, D., Molgo, J., Connold, A. L., Faille, L. The levator auris longus muscle of the mouse: a convenient preparation for studies of short- and long-term presynaptic effects of drugs or toxins. Neurosci Lett. 82, 83-88 (1987).
- Lanuza, M. A. Pre- and postsynaptic maturation of the neuromuscular junction during neonatal synapse elimination depends on protein kinase. C. J Neurosci Res. 67, 607-617 (2002).
- Garcia, N., Santafe, M. M., Tomas, M., Lanuza, M. A., Tomas, J. Short-term effects of beta-amyloid25-35 peptide aggregates on transmitter release in neuromuscular synapses. J Neuropathol Exp Neurol. 67, 250-259 (2008).
- Wright, M. C., Cho, W. J., Son, Y. J. Distinct patterns of motor nerve terminal sprouting induced by ciliary neurotrophic factor vs. botulinum toxin. J Comp Neurol. 504, 1-16 (2007).
- Wright, M. C. Distinct muscarinic acetylcholine receptor subtypes contribute to stability and growth, but not compensatory plasticity, of neuromuscular synapses. J Neurosci. 29, 14942-14955 (2009).
- Voss, A. A. Extracellular ATP inhibits chloride channels in mature mammalian skeletal muscle by activating P2Y1 receptors. J Physiol. 587, 5739-5752 (2009).
- Murray, L. M., Gillingwater, T. H., Parson, S. H. Using mouse cranial muscles to investigate neuromuscular pathology in vivo. Neuromuscul Disord. 20, 740-743 (2009).
- Dorje, F. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther. 256, 727-733 (1991).
- Caulfield, M. P., Birdsall, N. J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 50, 279-290 (1998).

