Electrolytic Inferior Vena Cava Model (EIM) of Venous Thrombosis
Summary
The electrolytic induction of endothelial activation to the internal surface of the Inferior Vena Cava results in venous type thrombus formation due to endothelial activation and partial blood stasis, two components of Virchow’s triad.
Abstract
Animal models serve a vital role in deep venous thrombosis (DVT) research in order to study thrombus formation, thrombus resolution and to test potential therapeutic compounds (1). New compounds to be utilized in the treatment and prevention of DVT are currently being developed. The delivery of potential therapeutic antagonist compounds to an affected thrombosed vein has been problematic. In the context of therapeutic applications, a model that uses partial stasis and consistently generates thrombi within a major vein has been recently established. The Electrolytic Inferior vena cava Model (EIM) is mouse model of DVT that permits thrombus formation in the presence of continuous blood flow. This model allows therapeutic agents to be in contact with the thrombus in a dynamic fashion, and is more sensitive than other models of DVT (1). In addition, this thrombosis model closely simulates clinical situations of thrombus formation and is ideal to study venous endothelial cell activation, leukocyte migration, venous thrombogenesis, and to test therapeutic applications (1). The EIM model is technically simple, easily reproducible, creates consistent thrombi sizes and allows for a large sample (i.e. thrombus and vein wall) which is required for analytical purposes.
Protocol
1. Mouse Anesthesia Procedures
- Animals are removed from their cage and placed in an anesthetic pre-operative induction chamber for gas induction at 2% isoflurane and 100% oxygen at a flow rate of 0.5 liters per minute (vaporizer controlled).
- Mice are sedated in the anesthetic induction chamber, removed from chamber and the ventral abdomen is shaved with electric clippers, or maintained within the chamber if a hair depilatory is used (1 min).
- While still under sedation, they are weighed, the eyes are lubricated with sterile opthalmic ointment and, then placed in dorsal recumbancy on a warm water circulating heating device under general anesthesia with a nose cone using isoflurane and oxygen. At this point the oxygen rate is reduced to 0.2 liters per minute.
- The abdomen is sprayed with chlorhexidine solution (chlorhexidine diacetate is rapidly bacterialcidal and persistent) and wiped with sterile gauze three times or until the surgical site is clean.
2. Mouse Micro-surgical and Recovery Procedures
- A ventral midline incision (2 cm) is made with iris scissors through the skin and abdominal wall exposing the abdomen. A sterile saline soaked 2×2 in gauze is used to reflect the intestines to the animal’s left side. A mouse restrainer is put in place allowing visualization of the inferior vena cava (IVC).
- At this point, the isoflurane is reduced to a maintenance level of 2% and the oxygen rate remains at 0.2 liters per minute for the remainder of the surgical procedure.
- Blunt dissection is facilitated using a sterile applicator swab and extra delicate iris half curved tissue forceps. Care must be taken in handling mouse tissues for they are extremely fragile.
- All IVC side branches, from the renal veins to the iliac bifurcation, are ligated with 7-0 non-reactive prolene sutures. Back branches remained patent.
- In the electrolytic IVC model (EIM), a 25G stainless-steel needle, attached to a silver coated copper wire (KY-30-1-GRN, Electrospec, Dover, NJ) is inserted into the exposed caudal IVC, (Figure 1) and positioned against the anterior wall (anode).
- Another wire is implanted subcutaneously completing the circuit (cathode).
- A current of 250 μAmps over 15 minutes was applied using a Grass S48 square wave stimulator and a constant current unit (Grass Technologies, An Astro-Med, Inc.,West Warwick, RI). The direct current results in the formation of toxic products of electrolysis that erode the endothelial surface of the IVC promoting a thrombogenic environment and subsequent thrombus formation.
- After 15 minutes, the needle is removed and a cotton swab is held gently in contact with the area to prevent bleeding
- In sham animals, the needle is placed into the IVC and then removed, without application of the current.
- The laparotomy site is closed in a two-layer fashion. Our laboratory uses 5-0 vicryl, a synthetic suture, in a continuous pattern to close the abdominal wall. Adhesive skin glue is used to close the skin. There are no external sutures for removal.
- Mice are then recovered in an individual mouse cage, closely observed post-operatively (45 minutes to 2 hours) under a heating lamp (min distance 24 inches away from cage), then returned to their original housing units. Pain medication and anti-inflammatory agents can be used if it does not interfere with study objective. Scientific justification to withhold pain medications, veterinary, and ICUCA approval are needed. Our laboratory has found that Buprenorphine,0.05-0.1 mg/kg SQ is an excellent pre- and post-operative analgesic for mice. In addition, Lidocaine at 4mg/kg (0.4 ml/kg of a 1% solution) can be infused in the incision site when systemic analgesics may confound experimental results.
- We typically do not experience post-operative adverse effects due to DVT. However, adverse post- operative effects can include spontaneous anesthetic death, surgical blood loss, and partial paresis due to nerve trauma. Animals that are slow to recover can be fed nutria gel or have moist chow placed on the floor of the cage to facilitate the animal’s recovery. Animals with sever post- operative complications should be humanely euthanized.
- At the assigned time point, euthanasia is performed humanely in accordance with the recommendations set forth by the American Veterinary Medical Association Guidelines on Euthanasia for rodents.
3. Representative Results
The EIM is a model that forms thrombus in the presence of continuous blood flow. The following figures show parameters investigated in this novel model.
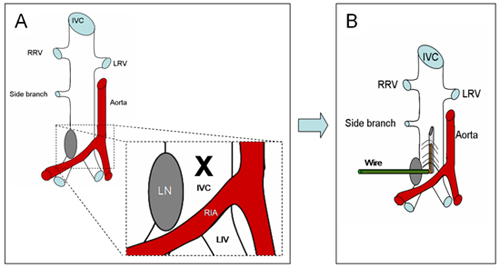
Figure 1 definesthe anatomical area in where a 25G stainless-steel needle, attached to a silver coated copper wire (KY-30-1-GRN, Electrospec, Dover, NJ) is inserted. Note that a lymphatic node is almost present and helps as a landmark to find the caudal IVC.
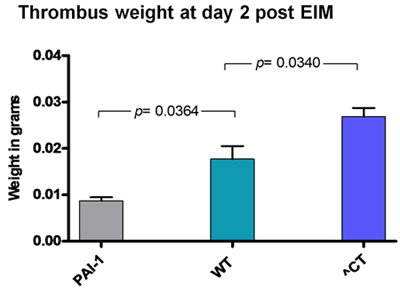
Figure 2: Thrombus weight 2 days after EIM in different strains. As expected, thrombus weight was lower in PAI-1 KO mice and larger in hypercoagulable Delta CT mice (^CT).
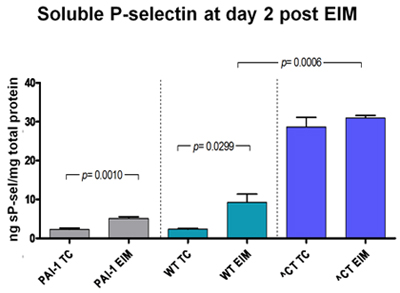
Figure 3: Soluble P-selectin, a maker for deep vein thrombosis, was measured in different strains. The lowest levels were found in PAI-1 KO mice whereas the highest levels were found in hypercoagulable delta CT mice.
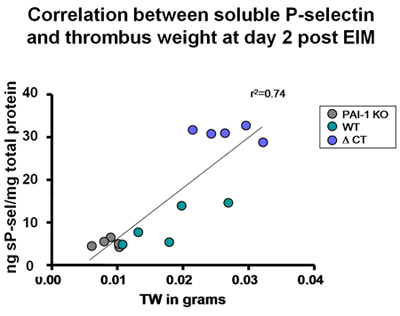
Figure 4 Correlation between thrombus weight and soluble P-selectin was found in different strains. Of note, in the group that generated the shorter thrombus size, the soluble P-selectin detected was the lowest (PAI-1 KO mice). In the group that generated the largest thrombus size, the soluble P-selectin detected was the highest (hypercoagulable delta CT).
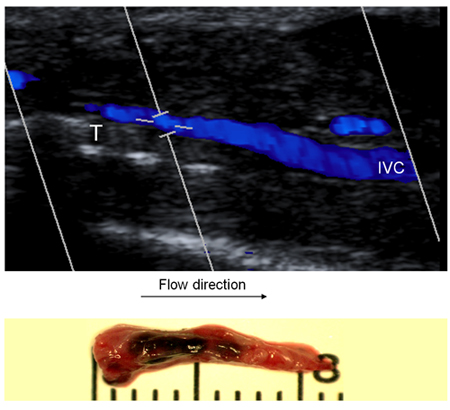
Figure 5: In order to demonstrate presence of blood flow, ultrasound was performed in mice undergoing EIM. Figure 5 shows the ultrasound performed in a mouse 2 days after EIM. Then the specimen was harvest. Note the that the thrombus shape suggest thrombus formation in presence of flow
Discussion
The mouse is an excellent research tool for in vivo experiments. Thus, the development of mouse models that closely mimic diseases or pathological conditions are necessary. Several venous thrombosis mouse models have been used to study DVT including: photochemical (2), stasis (3-4) and mechanical trauma (5-6). However, none of these models use an internal endothelial stimulus to generate thrombus in the presence of continuous blood flow. The EIM, using electrolytic stimulus within the IVC, promotes venous thrombogenesis by endothelial activation in the presence of blood flow (1). It is technically simple, easily reproducible and has a high survival rate (99%). Despite the fact that an electrical current is used to create the electrolysis, heat is not generated (1). The EIM model closely simulates clinical DVT, and therefore serves as a valuable tool for studying the intrinsic mechanisms of thrombus formation and resolution. The EIM model is also useful for the advancement of new therapeutic approaches against thrombosis disease.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
Supported by NIH 1P01HL089407-01A1 (Lawrence, PI), Animal Core A,NIH 1 K01 HL080962-01A2 (Myers, PI).
Materials
| Name of the reagent | Company | Catalogue number | Comments (optional) |
|---|---|---|---|
| DMEM | Invitrogen | ABCD1234 |
Referencias
- Diaz, J. A., Hawley, A. E., Alvarado, C. M. Thrombogenesis with continuous blood flow in the inferior vena cava. A novel mouse model. Thromb Haemost. 104, 366-375 (2010).
- Eitzman, D. T., Westrick, R. J., Nabel, E. G. Plasminogen activator inhibitor-1 and vitronectin promote vascular thrombosis in mice. Blood. 95, 577-580 (2000).
- Myers, D., Farris, D., Hawley, A. Selectins influence thrombosis in a mouse model of experimental deep venous thrombosis. J Surg Res. 108, 212-221 (2002).
- Myers, D. D., Hawley, A. E., Farris, D. M. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg. 38, 1075-1089 (2003).
- Pierangeli, S. S., Barker, J. H., Stikovac, D. Effect of human IgG antiphospholipid antibodies on an in vivo thrombosis model in mice. Thromb Haemost. 71, 670-674 (1994).
- Pierangeli, S. S., Liu, X. W., Barker, J. H. Induction of thrombosis in a mouse model by IgG, IgM and IgA immunoglobulins from patients with the antiphospholipid syndrome. Thromb Haemost. 74, 1361-1367 (1995).

