Isolation of Mouse Salivary Gland Stem Cells
Summary
An optimized protocol for the isolation of stem cells from the mouse salivary gland is described. The method employs enzymatic and mechanical digestion, and permits isolation of salispheres containing cells with characteristics of stem cells.
Abstract
Mature salivary glands of both human and mouse origin comprise a minimum of five cell types, each of which facilitates the production and excretion of saliva into the oral cavity. Serous and mucous acinar cells are the protein and mucous producing factories of the gland respectively, and represent the origin of saliva production. Once synthesised, the various enzymatic and other proteinaceous components of saliva are secreted through a series of ductal cells bearing epithelial-type morphology, until the eventual expulsion of the saliva through one major duct into the cavity of the mouth. The composition of saliva is also modified by the ductal cells during this process.
In the manifestation of diseases such as Sjögren’s syndrome, and in some clinical situations such as radiotherapy treatment for head and neck cancers, saliva production by the glands is dramatically reduced 1,2. The resulting xerostomia, a subjective feeling of dry mouth, affects not only the ability of the patient to swallow and speak, but also encourages the development of dental caries and can be socially debilitating for the sufferer.
The restoration of saliva production in the above-mentioned clinical conditions therefore represents an unmet clinical need, and as such several studies have demonstrated the regenerative capacity of the salivary glands 3-5. Further to the isolation of stem cell-like populations of cells from various tissues within the mouse and human bodies 6-8, we have shown using the described method that stem cells isolated from mouse salivary glands can be used to rescue saliva production in irradiated salivary glands 9,10. This discovery paves the way for the development of stem cell-based therapies for the treatment of xerostomic conditions in humans, and also for the exploration of the salivary gland as a microenvironment containing cells with multipotent self-renewing capabilities.
Protocol
1. Regent Preparation
- Buffer: 1 % (w/v) bovine serum albumin in Hank’s balanced salt solution
- Reconstitute enzymes. Hyaluronidase enzyme: 40mg / mL, dissolved in buffer. Collagenase II: 23mg / mL, dissolved in buffer. Use freshly prepared enzyme solutions fresh for each isolation. When dissolved, store at 4 °C until use for digestion.
- 50 mM calcium chloride in distilled water. Filter sterilize through a 0.2 uM pore size filter.
- Mouse salivary gland (MSG) culture medium: DMEM:F12 with penicillin (100 I.U. / mL), streptomycin (100 μg / mL), glutamax (2 mM), epidermal growth factor-2 (20 ng / mL), fibroblast growth factor-2 (20 ng / mL), N2 supplement (1 %), insulin (10 μg / mL) and dexamethasone (1 μM).
2. Mechanical and Enzymatic Tissue Digestion
- Weigh the dissected salivary glands.
- Chop glands into a homogenous pulp using sterile curved dissection scissors in a small petri dish.
- Collect minced tissue in 14 mL tubes, using 1mL of buffer per 80mg submandibular tissue. Rinse the petri dishes clean of tissue using some of the buffer.
- Add another 1 mL of buffer per 80 mg tissue, followed by 25 μL collagenase II enzyme solution, 25 μL hyaluronidase enzyme solution and 250 μL calcium chloride solution per 80 mg tissue. If working with large amounts of tissue, steps 2.4 – 2.9 can be performed in T25 tissue culture flasks for convenience.
- Incubate in a shaking waterbath set at 37 °C for 20 minutes. Remove tubes and triturate by pipette to mix enzyme thoroughly through tissue again.
- Replace in waterbath for another 20 mins.
- Collect tissue by centrifugation at 400 x g, for 8 minutes. Discard supernatant.
- Resuspend in 2 mL buffer for each 80 mg tissue, and repeat enzyme and calcium chloride addition as above. Incubate 20 minutes in shaking water bath. Remove tubes and triturate by pipette to mix enzymes thoroughly.
- Incubate for final 20 min in shaking water bath. Collect cells by centrifugation as above, discard supernatant.
3. Washing Steps
- Resuspend each 80 mg of tissue in 2 mL buffer and pipette to wash tissue free of enzymes.
- Centrifuge as previously to collect. Discard supernatant.
- Repeat wash using 1 mL buffer per 80 mg tissue. Centrifuge to collect, discard supernatant.
4. Filtering
- Resuspend tissue solution in 1 mL buffer per 80 mg tissue.
- Add solution to 100 μm pore-size filter placed over 50 mL Falcon tube. Do not apply more than 3 mLs of minced tissue solution per column, as filters may become blocked. Allow to seep through. Remove filtered material hanging on underside of filter by pipette, and add to filtrate.
- Use syringe with 26 gauge needle to take filtrate from 50mL tubes and apply to 50 μm pore size filters on 5 mL tubes. Allow to filter through, assisting by loosening lids if necessary.
- Centrifuge tubes as previously to collect. Discard supernatant.
5. Plating and Medium
- Combine all pellets into one volume. Count using automated cell counter or haemocytometer.
- Plate cells at density of 0.4 x 106 cells per well of 12-well plate, or 2.67 x 106 cells per T25 tissue culture flask. Add 1 mL MSG medium to each well or 6 mL to each T25 flask.
- Incubate at 37 °C. Spheres should be clearly visible by day 2.
6. Representative Results:
After two to three days in culture, small aggregates of cells (salispheres) will be apparent in the cultures. Salispheres will continue to grow in size over a period of ten days in culture. Representative phase contrast microscopy images of salispheres are shown in Figure 1. Proliferating cells expressing stem cell-associated marker proteins can be isolated from these spheres, optimally between days 3-5 post isolation, and are capable of differentiation into functional, saliva producing acinar cells.
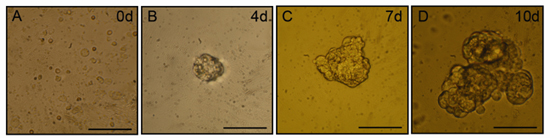
Figure 1. Salisphere formation in vitro. Following mechanical and enzymatic digestion using the present protocol, spheres of increasing size can be found in the floating cultures. Panels are representative phase contrast microscopy images of spheres from days 0 (A), 4 (B), 7 (C) and 10 (D). Scale bar = 50 μm.
Discussion
The tissue culture method described here represents a reproducible protocol for the isolation of stem cell-containing salispheres from the salivary glands of mice. Studies using cells isolated in this manner have highlighted the regenerative capacity of salivary gland stem cells 9. Transplantation of one hundred of c-Kit+ cells derived from the salispheres induced functional recovery of irradiated mouse salivary glands. These data are exciting and provide a starting point for the investigation of stem-cell based therapy for xerostomia. Many avenues remain to be explored however, including the full marker protein expression profile of the stem cells, the ability of submandibular glands to rescue the function of irradiated parotid salivary glands and vice versa, and the characterisation of the putative in vivo stem cell niche of the cells. Ultimately, the translation of this protocol to human tissue samples and the subsequent potential for the therapy of xerostomia in human patients using the isolated cells is the most exciting application of the described method.
Divulgaciones
The authors have nothing to disclose.
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Hyaluronidase | Sigma | H3506 | Store at – 20 °C | |
| Collagenase II | Gibco | 17101-015 | Store at 4 °C | |
| Epidermal Growth Factor-2 | Sigma | E9644 | Make a stock of 10 μg / mL in phosphate buffered saline (PBS). Store at – 20 °C in single use aliquots. | |
| Fibroblast Growth Factor-2 | Sigma | F0291 | Make stock of 25 μg / mL in PBS. Store at – 20 °C in single use aliquots. | |
| N2 supplement | Gibco | 17502-048 | As manufacturer instructions. | |
| Insulin | Sigma | I6634 | Make stock of 2 mg / mL in tap water. Adjust water to pH 2-3 using glacial acetic acid prior to dissolving. | |
| Dexamethasone | Sigma | D4902 | ||
| 100 μM pore-size sterile cell strainers | BD Falcon | 352360 | ||
| Polystyrene round-bottomed tubes with cell strainer caps (50 μM pore size) | BD Falcon | 352235 |
Referencias
- Vissink, A. Oral Sequelae of Head and Neck Radiotherapy. Crit Rev Oral Biol Med. 14, 199-212 (2003).
- Napeñ, a. s., J, J., Brennan, M. T., Fox, P. C. Diagnosis and treatment of xerostomia (dry mouth). Odontology. 97, 76-83 (2009).
- Denny, P. C. Parenchymal cell proliferation and mechanisms for maintenance of granular duct and acinar cell populations in adult male mouse submandibular gland. 235, 475-485 (2005).
- Man, Y. G. Persistence of a perinatal cellular phenotype in submandibular glands of adult rat. J. Histochem. Cytochem. 43, 1203-1215 (1995).
- Cotroneo, E., Proctor, G. B., Carpenter, G. H. Regeneration of acinar cells following ligation of rat submandibular gland retraces the embryonic-perinatal pathway of cytodifferentiation. Differentiation. 79, 120-130 (2010).
- Eirew, P. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med. 14, 1384-1389 (2008).
- Gorjup, E. Glandular tissue from human pancreas and salivary gland yields similar stem cell populations. European Journal of Cell Biology. 88, 409-421 (2009).
- Alonso, L., Fuchs, E. Stem cells of the skin epithelium. Proc Natl Acad Sci U S A. 100, 11830-11835 (2003).
- Lombaert, I. M. Rescue of salivary gland function after stem cell transplantation in irradiated glands. Plos One. 3, e2063-e2063 (2008).
- Coppes, R. P., Goot, A. v. a. n. d. e. r., Lombaert, I. M. A. Stem Cell Therapy to Reduce Radiation-Induced Normal Tissue Damage. Seminars in Radiation Oncology. 19, 112-121 (2009).

