Subdural Implantation of a Soft Electrocorticography Array for Cortical Electrophysiology Recording in Minipigs
Abstract
Source: Fallegger, F. et al., Subdural Soft Electrocorticography (ECoG) Array Implantation and Long-Term Cortical Recording in Minipigs. J. Vis. Exp. (2023).
This video demonstrates the implantation of a soft electrocorticography array and the long-term cortical recordings in a minipig. It outlines the steps involved in surgically implanting the array over the minipig's auditory cortex and recording both baseline and sound stimulus-evoked neuronal action potentials.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Surgical implantation of soft electrocorticography (ECoG) arrays
- Anesthesia
- Premedication: Isolate the Female Göttingen minipigs (n = 7) at 2-6 months of age (5-8 kg) and fast it overnight. Inject a mix of midazolam at 0.75 mg/kg, atropine at 0.25 µg/kg, and haldol at 0.1 mg/kg intradermally, and wait until the animal is sedated. Weigh the animal before proceeding.
- Installation of intravenous (IV) lead:
- Place the animal on the surgery table on a heating pad. Induce anesthesia by placing a face mask on the animal, using sevoflurane at 3%-3.5%.
- Place electrocardiogram leads on the abdomen, a blood saturation sensor on the tail, and a temperature sensor in the nostril.
- Place an IV lead on an ear vein and confirm blood access using a syringe filled with saline. Ensure the eyes are kept hydrated by using ointment.
- Intubation: Inject a bolus of atracurium at 0.5 mg/kg, ketamine at 1 mg/kg, and fentanyl at 1-2 µg/kg. Place the animal on its back for intubation. Insert a 4.5 mm tube.
- Medication: After intubation, stop the sevoflurane anesthesia and install a propofol infusion at 10 mg/kg/h, fentanyl at 2 µg/kg/h, atracurium at 0.2-0.5 mg/kg/h, and saline at 4-7 mg/kg/h. Start an infusion of mannitol at 1 g/kg/h to reduce brain swelling during the surgery.
NOTE: A multimodal analgesia regimen may be used if recommended by the local animal ethics committee.
- Aseptic field and skin preparation: Shave the entire surface of the head beyond the surgical field. Using a sterile pad, thoroughly scrub the head with betadine. Next, place sterile drapes on the instrumentation table and on the animal to reveal only the surgical window. Finally, scrub the head again with a sterile pad using betadine.
- Craniotomy and durotomy
- Skin cut: Incise the skin with a scalpel knife along the midline. Separate the muscle and periosteum (25 mm laterally from bregma on both sides and 40 mm anterior and posterior to bregma) from the bone using a raspatory and place spreaders to get optimum access for later drilling.
- Measurements and marking: Identify bregma and lambda and mark them with a sterile surgical pen (Figure 1A, B). Using a sterile ruler, define the bone flap outline centered around the implantation target on both hemispheres. In this specific case, the auditory cortex was chosen, with coordinates -5 mm to -15 mm from bregma and -4 mm to -20 mm laterally. Then, adjust the craniotomy to the size of the implant and anatomical landmarks, limiting the opening size.
- Craniotomy:
- Using a bone drill with a round cutting bit, drill the outline of the craniotomy, taking into account the thickness of the skull. Irrigate the drilling location with saline solution to avoid overheating the bone.
- Carefully drill the outline homogeneously until reaching the dura mater. At the first breakthrough, finish drilling the outline until it has thinned enough to almost break through. Then, use a flat spatula (either on the midline or the lateral side) to break away the bone flap in one piece, using the craniotomy edge as leverage. If too much resistance is encountered, continue thinning the bone.
- Place the bone piece in sterile saline.
- Once the bone flap is removed, carefully chip the edge of the craniotomy away, using a Kerison to avoid the sharp bone edge from cutting into the dura mater.
- If excessive bleeding is encountered on the dura mater or the bone, use Gelfoam or bone wax, respectively. Place a wet compress (standard pad in sterile saline solution) in the craniotomy and repeat this step on the other hemisphere (Figure 1B).
- Durotomy:
- Using the needle from a 6-0 suture kit, carefully pierce and lift the dura mater at the anterior or posterior end of the craniotomy halfway between the medial and lateral side and create the beginning of an incision with the stab knife.
- Then, using a small flat spatula inserted in the subdural space acting as a cutting base to protect the cortex, create an anteroposterior slit in the dura mater by advancing simultaneously with both tools. Ensure that the slit is slightly larger than the width of the implant (Figure 1C). If any bleeding or damage occurs at this step, cover it with gel foam and wait until it stops.
NOTE: The slit trajectory should be adapted if large blood vessels are present in the dura mater to avoid bleeding.
- Implantation
- Device insertion:
- Irrigate the implant (Figure 2A) with saline on both sides so it slides into the subdural space more easily. Place the implant above the dura mater slit and, with small forceps, subdurally insert the device by sliding it sequentially on each edge.
- Carefully hold the pedestal end of the device and advance with the implant in order not to create tension hindering the insertion. When the connector edge is located on top of the slit, stop the insertion.
- Secure the implant: To secure the implant in place, place a titanium bridge over the cable after the edge of the craniotomy or in the anchoring wings and secure it with one or two titanium screws using the appropriate screwdriver (Figure 1D).
- Ground placement: Carefully remove 1 cm of the insulation of the ground wires and insert it epidurally at the posterior end of the craniotomy (or any epidural location far from the cortex of interest or large blood vessels) (wire in Figure 1E)
- Repeat the steps on the contra-lateral hemisphere.
- Dura mater closure: Suture the dura mater carefully around the implant cable using a 3-0 resorbable suture and a small needle holder. Bring the two dura mater edges together as much as possible without tearing through the thin membrane with the suture wire (Figure 1D, E).
- Bone flap placement: Fix a titanium bridge on the anterior and posterior part of each bone flap using a titanium screw. Be careful to plan the positioning of the Ti-bridges with respect to the placement of the footplate legs in the next steps. Screw the end of the titanium bridges to the skull (Figure 1F, G).
- Device insertion:
- Pedestal and footplate placement
- Positioning: In this configuration, the footplate has six legs with two screw holes each (Figure 2B). Plan the placement of the footplate on the skull to optimize the location of the screws (avoid placing them at the edge of the craniotomy or in the temporal muscle). Skip the holes in the legs if they cannot be screwed in.
- Footplate securing: Screw in the titanium screws of the footplate until it is firmly in place (see Figure 1H).
- Pedestal placement: Remove the titanium bridges over the connection cables and flip over the pedestal to land on the footplate. Screw the pedestal onto the footplate. Check that the pedestal is firmly in place (Figure 1I).
- Suture and closure
- Cleaning of the wound: Clean the subcutaneous space of any bone or other debris by flushing with saline. Cut away some skin around the pedestal edges to create a round edge following the cylinder.
- Subcutaneous sutures: Remove the spreaders and fold the skin flaps together. Create subcutaneous sutures with a 4-0 non-resorbable suture wire, 3 mm apart, using simple interrupted sutures or simple continuous sutures. Start away from the pedestal, moving toward it on both sides of the incision.
- Dermal sutures: Suture the skin using a 6-0 non-resorbable suture wire, with sutures 5 mm apart. Start away from the pedestal, moving toward it on both sides of the incision. Take care to achieve good tissue apposition between the two skin flaps and near the pedestal edge to avoid a void (Figure 1J).
- Wound dressing: Clean the wound area again with a sterile pad and betadine. Apply a self-adhesive sterile bandage over the wound.
- Awakening: After all the measurements have been performed, take the animal off all anesthetics but keep under ventilation. For analgesia, apply a buprenorphine patch (25 mg/h) for 24 h. Place the animal on a heating pad covered with drapes to speed up the wake-up time. When spontaneous breathing is recovered, extubate the animal and put under an oxygen face mask until consciousness is recovered (which can take 1 to 4 h).
- Postoperative animal care: For 5 days, keep the animal under close surveillance. Give a dose of cephalexin at 75 mg twice daily with food, separated from other animals. Carry out disinfection of the wound daily by applying copious amounts of betadine with soaked sterile pads (best done during feeding).
NOTE: Long-term care and housing: The operated animal is kept isolated for 24 h. It is put back in its original social group if the animal is well enough to interact socially with its peers. Daily observation of the pedestal and skin opening needs to be conducted to follow the integration of the device on the head. When appropriate, clean the location around the pedestal with copious amounts of betadine.
2. Electrophysiological recording
- Spontaneous activity: Plug the wireless recording system through the pedestal and record the baseline activity for 2-3 min. These recordings will serve as a control to analyze the auditory evoked potentials.
- Auditory evoked potentials: In addition to the wireless system, insert speakers in a closed field in the animal ears. Play tone burst acoustic stimulation at different frequencies (ranging from 200-20,000 Hz) at around 70 dB sound pressure level (SPL) over 120 repetitions. Then, average the recordings and align them over the stimulus period for analysis.
- Sensory evoked potentials: Place the needles in the snout at three different positions. Evoke sensory potentials by stimulating the snout for ~30 s with the pulse generator at different amplitudes to obtain the recruitment curves.
3. Freely moving recording
- Follow the same procedure described in section 2 for recording awake signals from the brain. Plug the wireless headstage by either holding the animal in the experimenter's arms or feeding the animal with treats to distract it. Provide the acoustic stimulation using external speakers placed close to the animal.
Representative Results
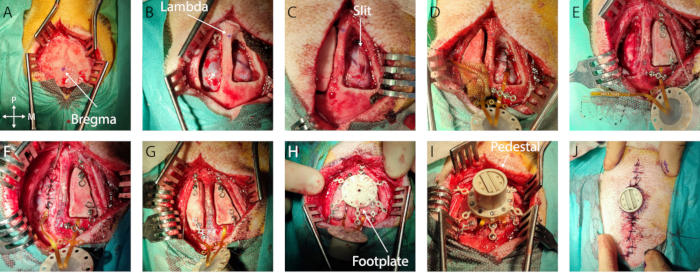
Figure 1: Minimally invasive implantation of soft ECoG onto the brain. (A) Surgical access to the skull, with indication of bregma. (B) Bilateral craniotomy with visible dura mater. (C) Slit durotomy on the first hemisphere. (D) Subdural implantation of soft ECoG and dura mater closure. (E) Slit durotomy on the second hemisphere. Bone flap fixation on the first hemisphere using titanium bridges. (F) Implantation of soft ECoG on the second hemisphere and dura mater closure. (G) Bone flap fixation on the second hemisphere. (H) Footplate positioning on the skull. (I) Pedestal fixation onto the footplate. (J) Skin closure around the pedestal base.
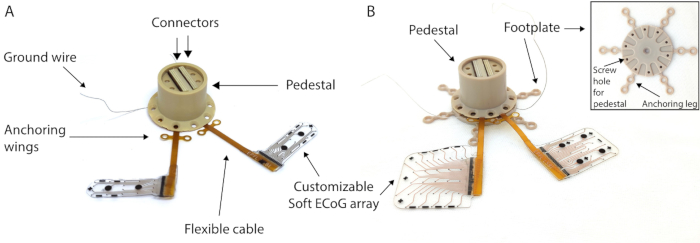
Figure 2: MRI-compatible pedestal. (A) Chronic MRI-compatible transdermal connection system (pedestal) to access the soft electrode array. (B) Pedestal with electrodes mounted on the footplate for skull anchoring. Inset: Details of the footplate.
Divulgaciones
The authors have nothing to disclose.
Materials
| Bone drill | BBraun | Elan 4 with GA861 handpiece | |
| Bone drill bit | BBraun | Neurocutter GP204R | |
| Bonewax | Ethicon | W31G | |
| Catheter | Venisystems | Abbocath 14G | |
| Gelfoam | Pfizer | Gelfoam | |
| Insert speakers | Etymotic | Etymotic ER2 insert Earphones | |
| Potentiostat | Gamry Instruments | Reference 600 | |
| Pulse Generator | AM Systems | Model 2100 Isolated Pulse Stimulator | |
| Recording headstage | Multichannel systems | W2100-HS32 | |
| Recording system | Multichannel systems | W2100 | |
| Screwdriver | Medtronic | Handle: 001201, Shaft: 8001205 | |
| Stab knife | Fine Science Tools | 10316-14 | |
| Suture wire dermal | Ethicon | Vicryl 2-0 | |
| Suture wire dura mater | Ethicon | Mersilk 5-0 | |
| Suture wire for lifting dura | Ethicon | Prolene 6-0 with BV-1 needle | |
| Suture wire subcutaneous | Ethicon | Vicryl 4-0 | |
| Titanium bridge | Medtronic | TiMesh 015-2001-4 | Cut out the required size |
| Titanium screws | Medtronic | 9001635, 9001640 | |
| X-ray system | GE | GE OEC 9800 Plus C-Arm |

