Installation of Optical Windows in the Brain of Transgenic Mice to Image Neural Activities
Abstract
Source: Lin, X., et al. Imaging Neural Activity in the Primary Somatosensory Cortex Using Thy1-GCaMP6s Transgenic Mice. J. Vis. Exp. (2019).
This video demonstrates the brain's neural activity recording through an optical window in transgenic mice. The mice express a fluorescent protein that enables the detection of neural activities. A small portion of the skull is carefully removed to install an optical window, and the skull is then covered with black cement to block light before imaging.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparing the Animal for Imaging
- Place sterile gauze, cotton swabs, and autoclaved surgical tools in the surgery area. Turn on a Micro Bead Sterilizer for intersurgery sterilization of surgical tools.
- Anesthetize the mouse (male or female, 1.5–2.5 months, 20–25 g) by intraperitoneal injection (i.p.) of a mixture of ketamine (17.2 mg/mL), xylazine (0.475 mg/mL) and acepromazine (0.238 mg/mL) (6 µL/g body weight). Wait until the proper depth of anesthesia is reached when the animal ceases to respond to a toe pinch stimulus and has no corneal reflex. During the surgery, give a booster dose of 1/3 of the original dose of the anesthetic cocktail as needed to maintain the original anesthetic state.
- Inject buprenorphine subcutaneously (s.c.; 0.05–0.10 mg/kg) as an analgesic agent prior to surgery. Apply protective ointment to the eyes of the animal to prevent corneal drying and cataract formation.
- Place a GCaMP6s transgenic mouse on a heating pad to prevent hypothermia and cover it with a sterile surgical drape. Stabilize the animal's head with a Head Holding Adaptor for Mice.
- Shave the head over most of the scalp with a double-edge razor blade. Clean the surgical area with a sterile alcohol preparation pad, followed by a povidone-iodine solution.
2. Chronic Cranial Window Preparation
- Make a rectangular cut of the scalp (2 mm x 3 mm) with a pair of scissors on one side (right) of the skull. Push the skin aside with a cotton swab to create an exposure area of >3 mm in diameter (Figure 1A). Remove the connective tissue attached to the skull by gently scraping the skull with a blunt microsurgical blade (Scalpel Blade #10; Figure 1C).
- Mark the S1 area by gently drawing a circle (3 mm in diameter, center coordination: -1.5 mm from the bregma and 2.5 mm from the midline) on the skull using a dental drill (Figure 2B).
- Perform the same procedure on the left skull (Figure 2B).
- Apply a thin layer of Cyanoacrylate Super Glue to the bone to provide a base for dental cement application.
- Under a dissecting microscope, thin down a circular groove in the skull around the S1 area using a high-speed microdrill (Figure 1D). The purpose is to create a smooth edge for the placement of an optical window onto it.
- Repeatedly apply artificial cerebrospinal fluid (ACSF, room temperature) to the skull periodically during the thinning process to facilitate drilling and dissipate heat. Perform the drilling intermittently to avoid friction-induced overheating. Suck away the bone debris with a house vacuum line or a vacuum pump.
- After approximately 2/3 depth of the bone is drilled, slowly and carefully thin the remaining 1/3 bone until a circular piece of the skull (bone flap) is completely free from the surrounding skull.
- Slowly and carefully remove the circular bone flap with a pair of # 5/45 forceps and expose the dura (Figure 2C; Figure 1E).
- Keep the exposed brain region moist with ACSF (Figure 1E). Avoid any damage to pial vessels since hemorrhage will alter cerebral blood flow, accelerate brain swelling, and severely degrade imaging quality.
- Perform the same procedure on the left (contralateral) side of the skull.
- For assembling the optical window, use optical adhesive to glue and cure between coverglass layers one at a time. If necessary, warm the optical window (50 °C for 12 h) in an incubator to enhance the adhesive bonding. The optical window contains 2 portions: the top portion contains a single round cover glass with a diameter of 5 mm and the bottom portion contains 1–3 round cover glasses with a diameter of 3 mm (Figure 2D).
- Store the optical window in ethanol (70%, vol/vol) and rinse it with sterile saline before use. Before optical window installation, check the optical window for imperfections, including an incorrect amount of optical adhesive or inaccurate alignment under a stereoscope.
- Install the optical window over the craniotomy region (Figure 2D). The top portion of the optical window rests on the skull, and the bottom portion of the optical window fits within the craniotomized opening and rests on the dura in the presence of CSF (Figure 2D, E).
- Seal the top portion of the optical window edge to the skull with the Cyanoacrylate Super Glue (Figure 2F; Figure 1F).
- When the Cyanoacrylate Super Glue has dried, apply black dental cement (Dentsply) to cover the glass edge. Apply dental cement to all the exposed skull and wound margins to block light (Figure 2G-H; Figure 1B).
- Perform the same procedure on the left side.
- Allow the animal to recover on the heating pad and return the animal to its home cage for recovery under appropriate monitoring. Provide wet food to facilitate chewing and hydration. To reduce pain, administer buprenorphine s.c. (0.05–2.0 mg/kg) every 8–12 h postinjury for 2 days. Allow the mouse to recover for an additional 7–10 days before imaging.
Representative Results
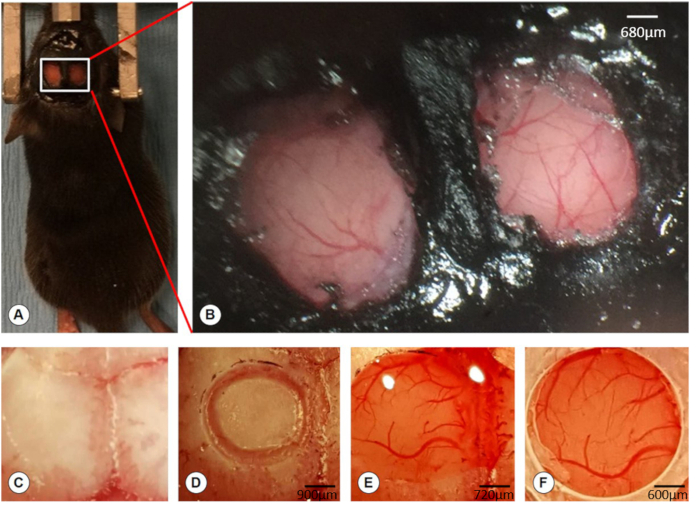
Figure 1. Creation of bilateral windows on the skull. (A) A photographic image shows 2 cranial optical windows on each side of the skull overlying the S1 area. (B) High magnification of the boxed region of the image (A) showing bilateral optical windows exposing the underlying dura and blood vessels. Scale bar = 680 µm. (C) The exposed skull. (D) Creation of a circular groove within which a central bone flap is seen. Scale bar = 900 µm. (E) After the removal of the bone flap, the cranial opening was filled with artificial cerebrospinal fluid (ACSF). Scale bar = 720 µm. (F) Installation of the optical window over the cranial opening and sealing the edge of the window with super glue. The dura and blood vessels are visible through the optical window. Scale bar = 600 µm.
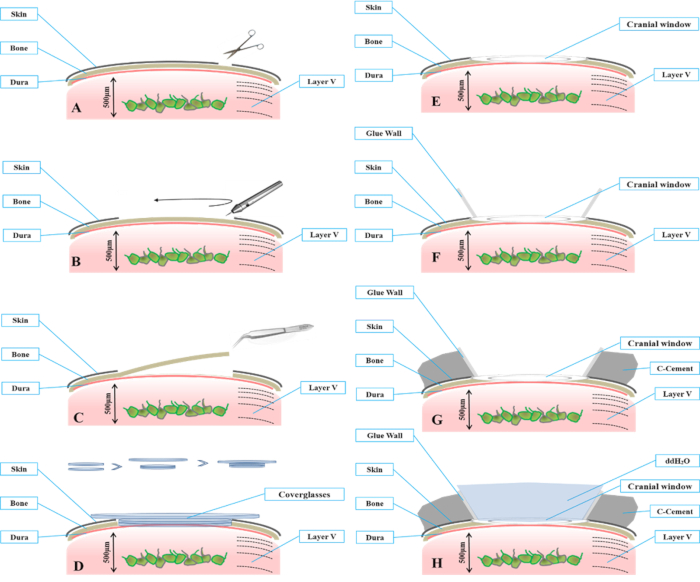
Figure 2: Schematic drawing shows key steps for preparing a cranial window. (A) Under a dissecting microscope, remove the skin over the surgical region using a pair of surgical scissors. (B) Use a high-speed microdrill to produce a circular groove (3 mm diameter) on the skull over the S1 area until the bone flap within the groove becomes detached from the surrounding bone. (C) Slowly remove the bone flap to expose the underlying dura. (D) Assemble the optical window with coverglasses with 2 different diameters. The upper coverglass has a larger diameter (5 mm), and the lower coverglasses (usually 1-3) have a smaller diameter (3 mm). The thickness of the coverglasses to be installed will be dependent on the thickness of the skull. (E) Place the coverglass onto the circular opening of the skull to create an optical window. The lower portion of the coverglasses should fit within the cranial opening. The bottom of the coverglasses should gently contact the dura. (F) Seal the window edges to the skull with cyanoacrylate glue. (G) When the cyanoacrylate dries, apply black dental cement lateral to the glue wall. (H) Fill the cranial window with ddH2O and use water-immersion objectives for imaging.
Divulgaciones
The authors have nothing to disclose.
Materials
| C57BL/6J-Tg(Thy1-GCaMP6s)GP4.3Dkim/J | Jackson | 24275 | Can be any strains made by the genetically-encoded neuronal indicator and effector expressing the green fluorescent calcium indicator, GCaMP, in subsets of excitatory neurons in the cortex. |
| B6.Cg-Tg(Thy1-EGFP)OJrs/GfngJ. | Jackson | 7919 | Can be any strains expressing EGFP in subsets of neurons within specific populations in the cortex; providing a bright, vital Golgi-like stain. |
| Dumont no. 5 forceps, standard, Dumoxel | Fine Science Tools | 11251-30 | Can be any brand of choice. |
| Dumont no. 5SF forceps | Fine Science Tools | 11252-00 | Can be any brand of choice. |
| Micro Bead Sterilizer | Southern Labware | B1201 | Can be any brand of choice. |
| Harishige's SG-4N Head Holding Adaptor | Tritech Research | SG-4N | Can be any brand of choice. |
| Ideal Micro-Drill Complete Kit | Harvard Apparatus | 72-6065 | Can be any brand of choice. |
| Burrs for Micro Drill | Fine Science Tools | 19007-05 | Can be any brand of choice. |
| Round cover glass, 3 mm | Harvard Biosciences | W4 64-0720 | Can be any brand of choice. |
| Round cover glass, 5 mm | Harvard Biosciences | W4 64-0700 | Can be any brand of choice. |
| Norland optical adhesive | Norland Products | 7106 | Can be any brand of choice. |
| Loctite liquid super glue | WB Mason | LOC1647358 | Can be any brand of choice. |
| Grip cement kit, powder and solvent | Dentsply | 675570 | Can be any brand of choice. Mix 5 parts of white dental cement powder (Grip Cement) with one part of black Tempera powder paint (to shield from light; some users prefer a 10:1 ratio). |
| Gel foam | Moore Medical | 2928 | Can be any brand of choice. |
| Micro dissecting scissors | Roboz | RS-5841 | Can be any brand of choice. |
| Artificial cerebrospinal fluid (ASF) | – | – | The ACSF contains 125 mM NaCl, 4.5 mM KCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 2 mM CaCl2, 1 mM MgCl2 and 20 mM glucose. Bubble the ACSF with oxygen (95% oxygen, 5% carbon dioxide) and adjust the pH to 7.4. Prepare fresh ACSF solution before every experiment. |

