Utilizing Multielectrode Arrays to Measure Synaptic Physiology in 3D Coculture Spheres
Abstract
Source: Cvetkovic, C., et al. Synaptic Microcircuit Modeling with 3D Cocultures of Astrocytes and Neurons from Human Pluripotent Stem Cells. J. Vis. Exp. (2018)
This video demonstrates the procedure of using the multielectrode array (MEA) to measure synaptic physiology in 3D coculture spheres. This method allows to understand the neural network dynamics and synaptic network burst activity within the 3D spheres.
Protocol
Measurement of Live Synaptic Physiology with Multielectrode Arrays (MEAs)
1. Prepare MEAs for cell culture.
- Clean the surface of each MEA with 1 mL of detergent for 1 h. Rinse with sterile deionized (DI) water, rinse with 70% ethanol to sterilize, and then air dry in a biosafety cabinet under UV light.
NOTE: MEAs can be stored in DI water at 4 °C under sterile conditions until use. - Prepare a 0.5 mg/mL solution of poly-ornithine (PLO) by dissolving 50 mg of PLO into 100 mL of boric acid buffer. Coat the surface of each MEA with 1 mL of PLO to render it hydrophilic and incubate at 37 °C for a minimum of 4 h. Remove the PLO and wash the surface with DI water.
- Dilute extracellular matrix (ECM) coating solution with basal (DMEM/F12) media to prepare a 1 mg/mL stock solution. For a working solution, resuspend 3 mL of ECM stock into 33 mL of pre-chilled DMEM/F12 media, bringing the total volume to 36 mL for a concentration of 80 µg/mL.
- Add 1 mL of ECM working solution to each MEA and incubate at 37 °C for a minimum of 4 h.
- Remove the ECM and place the spheres in 1.5 mL of media on a MEA surface, ensuring that the spheres are positioned on top of the electrode array (Figures 1A, 1A'). Allow the media to adhere for 2 days. Change half of the media every 2–3 days (or more often if needed), ensuring that the spheres are not disturbed.
NOTE: The addition of growth factors may improve culture survival and maturation.
2. Measure the electrical activity of neural spheres.
- Set up a multielectrode array (MEA) system with a temperature-controlled headstage. Create a software program with a 5 Hz high-pass filter, a 200 Hz low-pass filter, and a spike threshold of 5x standard deviation (SD).
- Place the MEA on headstage and record spontaneous electrical activity (Figure 1B, B'). Save raw data, including spike frequency and amplitude, for statistical analysis.
NOTE: A perfusion system may be utilized with the MEA to add pharmacological agents or to increase the flow of fresh media for long-term recording. As desired, additional post-processing of spikes may be performed.
Representative Results
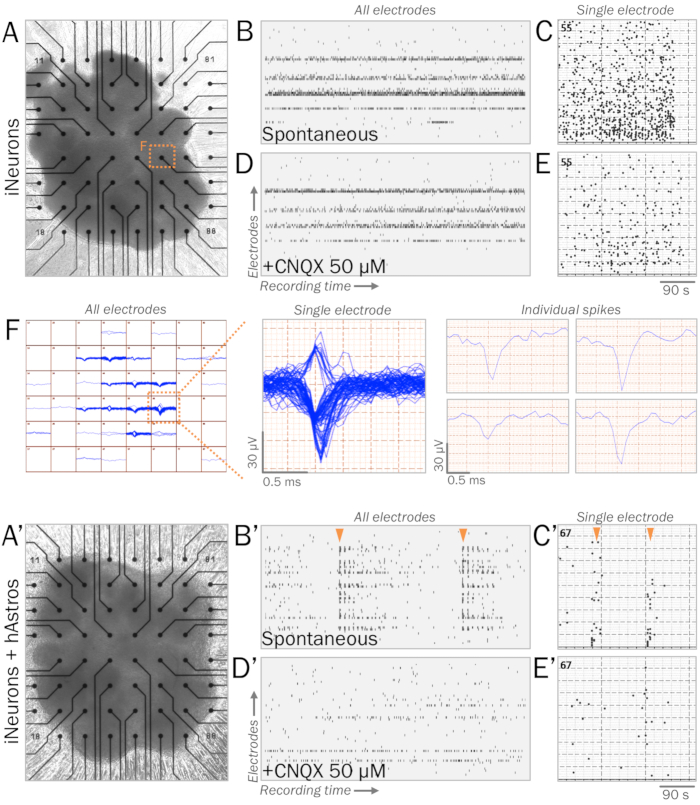
Figure 1: Representative live synaptic physiology of neural spheres on multielectrode arrays (MEAs). (A, A') MEAs are utilized to measure the live synaptic physiology of neural spheres. Spheres of iNeurons (A) or cocultures of iNeurons and hAstros (A') can be cultured on MEA surfaces with ECM-mimicking substrates (see step 4.1). (B, B') Raster plots of representative spontaneous electrical activity measured across all electrodes (vertical axis) during a recording window (horizontal axis). After 4 weeks of culture in neurophysiological basal medium41, iNeuron spheres (B) displayed spontaneous spikes, while coculture spheres (B') revealed increased network bursts of synchronous firing patterns (orange arrows). (C, C') Histogram of spikes measured during MEA recording windows from representative electrodes. (D, D') Raster plots of spontaneous electrical activity were measured across all electrodes after the application of 50 µM CNQX, an AMPA receptor antagonist (same time scale as B, B'). CNQX eliminated the presence of synchronous bursts of spikes observed in cocultures (D'). (E, E') Histogram of spikes measured during MEA recording windows from representative electrodes after 50 µM CNQX application (same time scale as C, C'). (F) Map of spike tracings of iNeuron spheres from all electrodes over time (left). Demonstrative single electrode displaying multiple clustered traces over time (middle); individual spike traces can be sorted and analyzed individually (right).
Divulgaciones
The authors have nothing to disclose.
Materials
| DMEM/F12 | Thermofisher | 10565-042 | With GlutaMAX supplement |
| Anti/anti | Thermofisher | 15240062 | |
| B27 | Thermofisher | 17504044 | Media Supplement |
| Heparin | Sigma | H3149-50KU | Working concentration: 2 mg/mL |
| Matrigel membrane matrix | Corning | 354230 | ECM coating solution. Working concentration: 80 ug/ml. Prepare on ice and ensure that pipettes, tubes, and media are pre-chilled. |
| MEA 2100 System | Multichannel Systems | MEA2100 | |
| N2 | Thermofisher | 17502048 | Media Supplement |
| Poly-l-ornithine (PLO) | Sigma | P3655-100MG | Working concentration: 0.5 mg/mL |

