Development and Maintenance of Serum-Free Embryoid Bodies
Abstract
Source: Phillips, A. W., et al. Developing HiPSC Derived Serum Free Embryoid Bodies for the Interrogation of 3-D Stem Cell Cultures Using Physiologically Relevant Assays. J. Vis. Exp. (2017).
The video describes the procedure for developing serum-free embryoid bodies (SFEBs) from neuronal progenitor cells using various serum-free differentiation mediums or DM. DM-2 facilitates neurons' maturation and arrangement to form SFEBs, while DM-3 is used for further growth maintenance.
Protocol
1. Serum-Free Embryoid Body (SFEB) Induction (Figure 1)
- Place 40 µm cell culture inserts into 6-well plates and add 1 mL differentiation medium 1 (DM1) (Table of Materials) at least 1 h before adding aggregates.
- Transfer the neural progenitor cell aggregates with 20 µL medium using a 200 µL wide mouth tip onto 40 µm cell culture inserts and change the medium to DM2 (Table of Materials).
NOTE: Cell culture inserts are specialized inserts that sit slightly off the bottom of the well and consist of a polytetrafluoroethylene membrane suspended across a plastic frame. This membrane is biocompatible and can efficiently sustain nutrient and oxygen transport to the SFEBs placed on top. They are commonly used with organotypic hippocampal slice cultures taken from a mouse or rat.- Transfer 4-6 aggregates to one cell culture insert and allow adequate space between the aggregates. Remove as much excess solution as possible using a fine pipette tip (e.g., 200 μL tip).
NOTE: It is acceptable to briefly disturb the SFEB as they will move on the cell culture insert as the solution is removed from around the SFEBs. - Maintain the cultures in 1 mL of DM2 at 37 °C, 95% humidity and 5% CO2. Using a pipette, change 75% of the medium every other day by removing three-quarters of the medium and replacing it with three-quarters fresh medium. For example, remove 750 μL of medium and add 750 μL of fresh medium.
- Transfer 4-6 aggregates to one cell culture insert and allow adequate space between the aggregates. Remove as much excess solution as possible using a fine pipette tip (e.g., 200 μL tip).
- After 14 days of culture, switch to DM3 medium (Table of Materials) with 75% medium change every other day and for another 16 days.
- To maintain the SFEBs on cell culture inserts beyond 30 days, change the medium every other day for 60, 90, and 120 days.
NOTE: The SFEBs will grow to about 1,000 μm in diameter and are typically 100-150 μm thick. - Detach the SFEBs from the insert by gently pipetting the medium using a 200 µL wide-mouth pipette tip. To transfer the SFEBs, use a wide-mouth pipette tip to gently suction the bodies into the pipet tip. After loading SFEB into the pipet tip, gently transfer to 12-well plates and wash with 300 μL phosphate-buffered saline (PBS) per well.
Representative Results
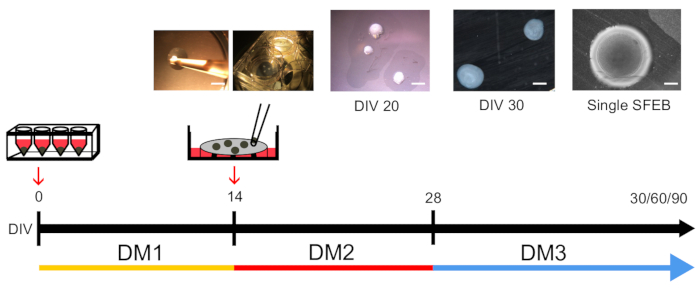
Figure 1: Timeline of Medium Changes and Phases during SFEB Growth and Expansion.
After SFEBs have been transferred to inserts, they undergo two medium changes at 14 and 28 days in vitro (DIV). They are typically harvested for experiments at DIV 30, 60, and 90. Upper insets show different phases in this process. Scale bars = 1,000 μm (far left); 1,000 μm (DIV 20); 600 μm (DIV 30); 300 μm (single SFEB).
Divulgaciones
The authors have nothing to disclose.
Materials
| SFEB Neuronal Differentiation Cell culture Media. Reagents. Components | |||
| STEMdiff Neural Induction Medium (hiPSC Media) | STEMCELL Technologies | 0-5835 | 250ml |
| PluriQ ES-DMEM Medium (MEF Media) | GlobalStem | GSM-2001 | |
| DM1 Media Components | |||
| D-MEM/F-12 (1X), Glutamax liquid, 1:1 | Invitrogen | 10565018 | 385ml |
| Knockout Serum Replacement | Invitrogen | 10828028 | 20% 100ml |
| Pen/Strep | Invitrogen | 15140122 | 5ml |
| Glutamax 200mM | Invitrogen | 35050061 | 5ml |
| MEM Non-Essential Amino Acids Solution 10 mM (100X), liquid | Invitrogen | 11140050 | 5ml |
| 2-Mercaptoethanol (1,000X), liquid | Invitrogen | 21985023 | 900ul |
| DM2 Media Components | |||
| D-MEM/F-12 (1X), Glutamax liquid, 1:1 | Invitrogen | 10565018 | 500ml |
| Glutamax 200mM | Invitrogen | 35050061 | 5ml |
| Pen/Strep | Invitrogen | 15140122 | 5ml |
| N-2 Supplement (100X), liquid | Invitrogen | 17502048 | 10ml |
| DM3 Media Components | |||
| NEUROBASAL Medium (1X), liquid | Invitrogen | 21103049 | 500ml |
| B-27 Supplement Minus Vitamin A (50X), liquid | Invitrogen | 12587010 | 10ml |
| Glutamax 200mM | Invitrogen | 35050061 | 5ml |
| Pen/Strep | Invitrogen | 15140122 | 5ml |
| Components/Materials | |||
| Millicell | PICM0RG50 | ||
| Phosphate Buffered Saline (PBS) | ThermoFisher | 10010023 | 500 mL |

