Preparing Rhinal Cortex-Hippocampus Slices from a Rat Pup Brain
Abstract
Source: Valente, C. A., et al., A Model of Epileptogenesis in Rhinal Cortex-Hippocampus Organotypic Slice Cultures. J. Vis. Exp. (2021)
The video demonstrates a method for obtaining rhinal cortex-hippocampus slices from a rat pup's brain and culturing them in membranous inserts within a multi-well plate filled with media for further analysis.
Protocol
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review committee.
1. Preparation of rhinal cortex-hippocampus slices
NOTE: The preparation of rhinal cortex-hippocampus slices uses P6-7 Sprague-Dawley rats.
- Culture setup and medium preparation
- On the day before the culture, prepare the required media and place them at 4 °C.
- Prepare dissection medium: 25 mM glucose in Gey's Balanced Salt Solution (GBSS).
- Prepare culture medium: 50% Opti-MEM, 25% HBSS, 25% Horse Serum (HS), 25 mM glucose, 30 µg/mL Gentamycin.
- Prepare maintenance medium: Neurobasal-A (NBA), 2% B27, 1 mM L-glutamine, 30 µg/mL Gentamycin, HS (15%, 10%, 5% and 0%).
- Brain harvesting
- Just before starting the culture, add 1.1 mL of culture medium to each well of the 6-well plate with a P1000 pipette and place it at 37 °C.
- Place all the equipment (dissection microscope, tissue chopper, dissecting lamp, dissecting tools, electrodes, plates, inserts, and filter papers) inside the biological safety cabinet and sterilize under UV light for 15 minutes.
- Adjust slice thickness to 350 µm.
- Withdraw the GBSS from the fridge. Add 5 mL of GBSS to six Petri dishes. Six Petri dishes will be required per animal.
- Euthanize the rat pup. Perform decapitation by using a sharp scissor at the base of the brain stem of the animal.
- Wash the animal head three times in cold GBSS and take it inside the safety cabinet.
- Tissue isolation and preparation of slices
- Firmly insert sharp forceps into the eye sockets to hold the head.
- Using a thin scissor cut the skin/scalp along the midline starting from the vertebral foramen towards the frontal lobes and put it aside.
- Cut in the same way as the skull and along the cerebral transverse fissure (space between the brain and cerebellum). With curved long forceps, move it apart.
- Discard the olfactory bulbs with a spatula. Remove the brain from the head and place it in ice-cold GBSS with the dorsal surface facing up (Figure 1A).
- Insert the fine forceps into the cerebellum and go along the midline with the spatula opening each hemisphere very carefully (Figure 1B).
- With short curve forceps, carefully remove the excess tissue that covers the hippocampi, without touching the hippocampal structure. Then with a spatula, cut below each hippocampus (Figure 1C).
- Pick up one hemisphere and place it, with the hippocampus facing up, onto a filter paper. Repeat the procedure with the other hemisphere and place it parallel to the first one, in the filter paper. Put the filter paper on the tissue chopper, with the hemispheres perpendicular to the blade, and cut the hemispheres into 350 μm slices (Figure 1D).
- Place the sliced tissue into a Petri dish with cold GBSS (Figure 1E).
- Carefully separate the slices using the round tip electrodes (Figure 1F). Keep only the slices with a structurally intact rhinal cortex and hippocampus. DG and CA areas should be perfectly defined, as well as the entorhinal and perirhinal cortex (Figure 1G).
- Place each slice onto the insert (Figure 1H-I), with a spatula and a round-tip electrode. Remove the excess dissection medium around each slice with a P20 pipette (Figure 1J). Four rhinal cortex-hippocampus slices can be cultured in a single insert (Figure 1K).
- Culture maintenance
- Change the medium every other day.
- Warm up the medium at 37 °C.
- Take the plates from the incubator. Pick up each insert by holding the plastic edge with forceps (Figure 1L).
- Use a free hand to aspirate the medium from the well. Place the insert back into the well and add 1 mL, with a P1000 pipette, of fresh warmed medium (Figure 1M). Repeat for all the inserts. Make sure no air bubbles are trapped between the membrane and the medium.
Representative Results
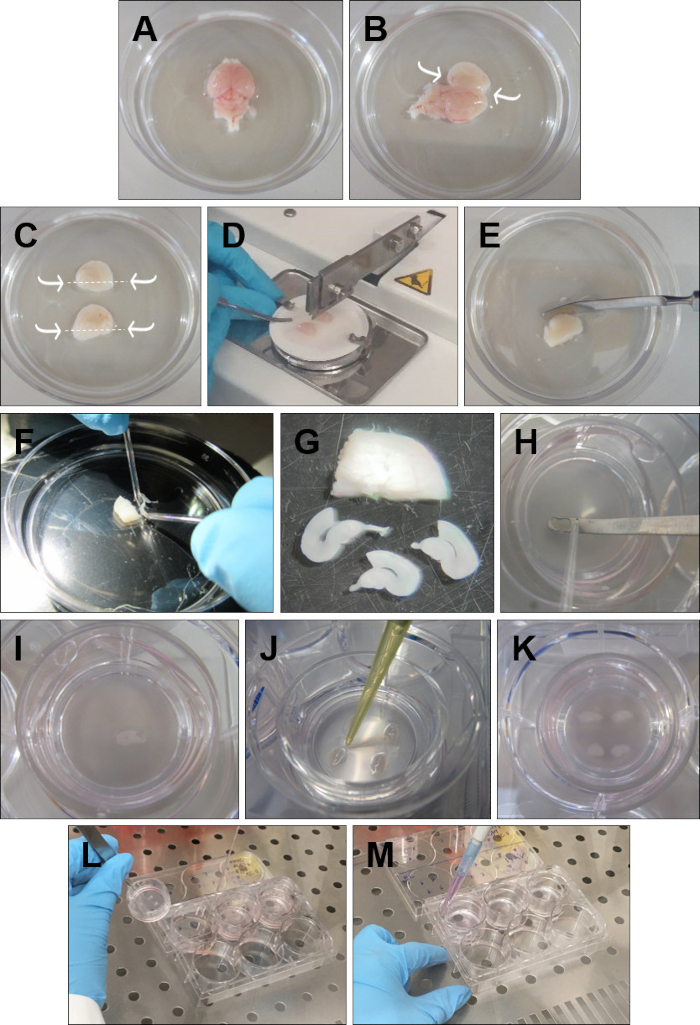
Figure 1: Detailed procedure for the preparation of rhinal cortex-hippocampus organotypic slices. (A) Remove the brain from the head and place it in ice-cold GBSS with the dorsal surface faced up. (B) Insert the forceps into the cerebellum. Open the brain through the midline and remove the excess tissue over the hippocampus. (C) With a spatula cut below the hippocampus, as indicated by the arrows. (D) Place both hippocampi facing up and parallel to each other onto the filter paper and cut 350 µm slices on the tissue chopper. (E) Place the sliced hippocampus in ice-cold GBSS. (F) Separate the slices with the help of round tipped glass electrodes. (G) Choose only the slices that depict an intact rhinal cortex and hippocampus. (H, I) With the help of a round tipped glass electrode push each slice to the spatula and place it on the insert. (J) Remove the GBSS surrounding the slice. (K) Place four slices per insert. (L) To change the medium, lift the insert and aspirate the medium with a glass pipette. (M) Add fresh medium by placing the pipette between the insert and the walls of the 6-well plate. Make sure there are no air bubbles beneath the slices.
Divulgaciones
The authors have nothing to disclose.
Materials
| Blades for scalpel handle | Fine Science Tools | 10011-00 | |
| Brain/Tissue Slice Chamber System | Warner Instruments | ||
| Cell culture inserts, 30 mm, hydrophilic PTFE | Millipore SAS | PICM03050 | |
| Conventional incubator | Thermo Scientific Heraeus | BB15, Function Line | Set to 37 °C and 5% CO2 |
| D(+)-Glucose monohydrate | Merck Millipore | 1.08342.1000 | |
| D-(+)-Glucose solution, 45% in water | Sigma | G8769 | |
| di-Sodium hydrogen phosphate dihydrate | Merck Milipore | 1.06580.1000 | |
| Dumont #5 Fine Forceps Biologie Inox | Fine Science Tools | 11254-20 | |
| Dumont #5 Forceps Standard Inox | Fine Science Tools | 11251-20 | |
| Dumont #7 Forceps Standard Dumoxel | Fine Science Tools | 11271-30 | |
| Dumont Medical #7S Forceps Short Curve Inox | Fine Science Tools | 11273-22 | |
| Gentamycin stock solution, 50 mg/mL | Thermo Fisher Scientific | 15750-037 | |
| Gey's Balanced Salt Solution (GBSS) | Biological Industries | 01-919-1A | |
| Glass Electrodes | Science Products | GB150F-10 | Round tips homemade |
| Glass Pasteur pipettes, 230 mm | VWR International | 612-1702 | |
| Horse Serum, Heat Inactivated (HS) | Thermo Fisher Scientific | 26050-088 | |
| Iris Spatula Curved | Fine Science Tools | 10092-12 | |
| Labculture Class II Biological Safety Cabinet | HERASafe | HS 12 | |
| L-Glutamine solution 200 mM (Q) | Thermo Fisher Scientific | 25030-024 | |
| Neurobasal-A Medium (NBA) | Thermo Fisher Scientific | 10888-022 | |
| Opti-MEM® I Reduced-Serum Medium | Thermo Fisher Scientific | 31985-047 | |
| Platinum 5 blades | Gillette | ||
| Qualitative Filter Paper, Cellulose, Grade 1, 55 mm | Whatman | 1001-055 | Medium retention 11µm |
| Qualitative Filter Paper, Cellulose, Grade 1, 90 mm | Whatman | 1001-090 | Medium retention 11µm |
| Scalpel handle | Fine Science Tools | 91003-12 | |
| TC-Treated Sterile 6-Wells Plates | Corning | CORN3516 | |
| Temperatue controller | MEDICAL SYSTEMS CORP. | TC-102 | |
| Tissue Chopper | The Mickle Laboratory Engineering CO. LTD. | MTC/2 | Set to 350 μm |

