Analyzing Neural Stem Cell Reactivation in Cultured Drosophila Brain Explants
Abstract
Source: Naomi Keliinui, C., et al. Neural Stem Cell Reactivation in Cultured Drosophila Brain Explants. J. Vis. Exp. (2022).
This video demonstrates a protocol to identify exogenous factors that initiate the reactivation of Drosophila brain neural stem cells from their quiescent state. Freshly hatched larvae are dissected to isolate their brains, which are then cultured in media with or without a test hormone to evaluate the hormone's potential to induce neural stem cell reactivation and proliferation.
Protocol
1. Culture media and tool preparation
- Spray the bench and work area with 70% ethanol and let dry.
- Spray the dissection tools, forceps, and two glass watch dishes, with 70% ethanol and let them dry on the bench.
- Make the supplemented Schneider's media (SSM, Table 1) and place it on ice.
- Pipette 1 mL of SSM into each of the glass watch dishes.
- Using a micropipette with a sterile tip, transfer the freshly hatched larvae from the plate of PBS to the SSM in the first glass watch dish. Using a micropipette with a sterile tip, transfer the freshly hatched larvae to the SSM in the second glass watch dish.
2. Dissections and brain cultures
- Once the larvae are in the second glass watch dish with SSM, dissect the brains out of the larvae using forceps and a dissecting microscope. Adjust the magnification as needed.
- Use one forceps to grab the mouth hooks and with the other, gently grab the body halfway down and pull in the opposite direction (Figure 1) to split the larva into two pieces.
NOTE: The brain will be located right behind the mouth hooks. Note that there may be other tissues surrounding the brain. Be very careful when removing these tissues as it can result in damaging the brain. - Once 15-20 brains have been dissected, add 1 mL of SSM into one well of a sterile 12-well culture tray. Transfer the freshly dissected brains into the SSM using a micropipette and a sterile tip (Figure 2A).
- Place the brains in the SSM media in the 12-well culture tray into an incubator at 25 °C for 24 h (Figure 2A).
Table 1: Recipe for making supplemented Schneider's media (SSM). The table lists the volume of all ingredients required for preparing 5 mL of SSM.
| Ingredient (starting concentration) | To make 5 mL | Final concentration |
| Schneider's Drosophila media | 3.89 mL | |
| Fetal bovine Serum (100%) | 500 µL< | 10% |
| Glutamine (200 mM) | 500 µL | 20 mM |
| Penicillin (5000 units/mL), Streptomycin (50,000 µg/mL) | 100 µL | 1000 U/mL Pen, 1 mg/mL Strep |
| Insulin (10 mg/mL) | 10 µL | 0.02 mg/mL |
| glutathione (50 mg/mL) | 5 µL | 0.05 mg/mL |
| In a sterile hood, using sterile technique, add all ingredients together in a 14 mL conical tube. Place it on ice until use. | ||
Representative Results
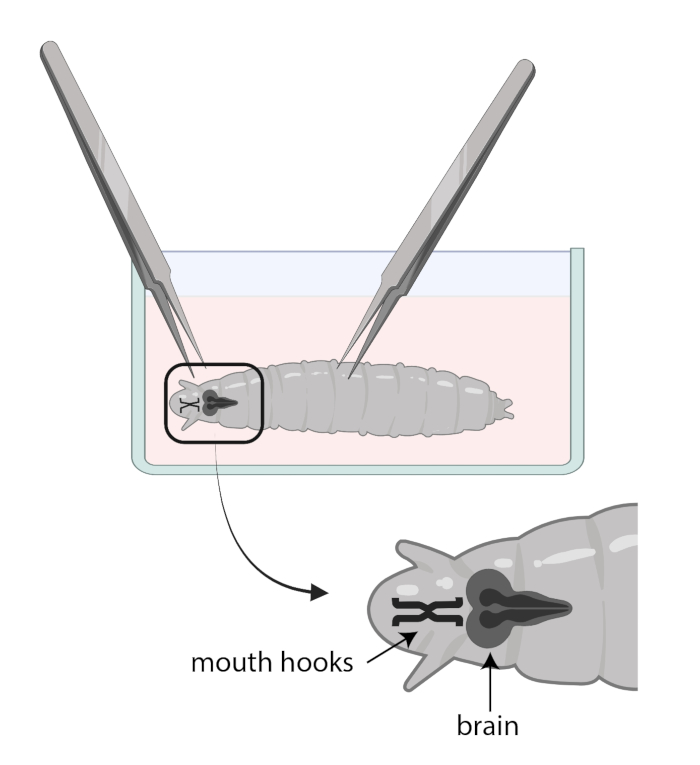
Figure 1: Drosophila larvae in a glass watch dish with SSM. Forceps are properly positioned for dissection. The location of the larval brain (dark grey) is posterior to the mouth hooks (black), and both are shown inside the larva.
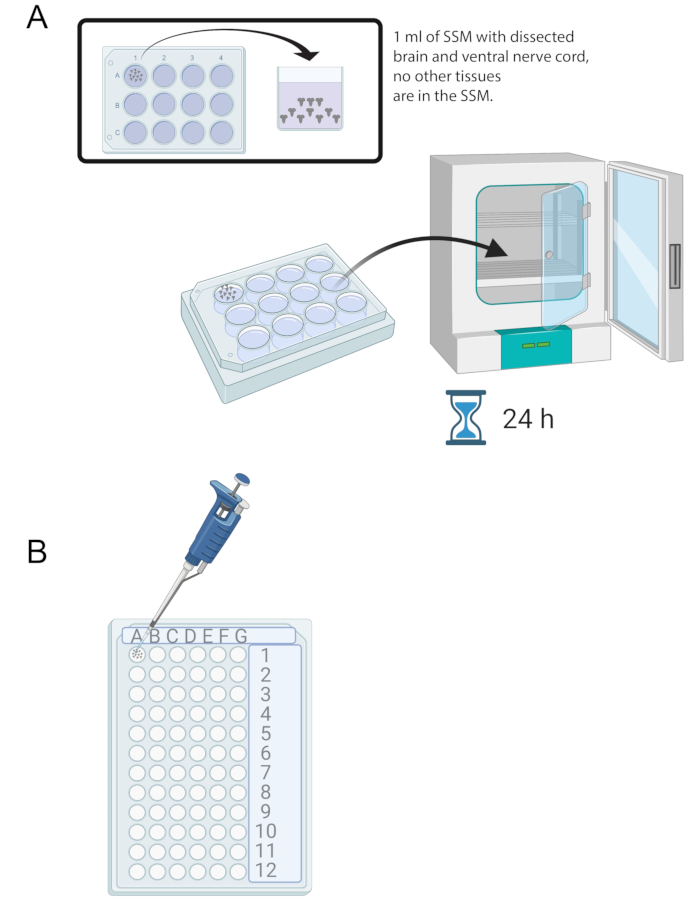
Figure 2: Brain culture and immunostaining. (A) Whole brains in a 12-well culture dish containing 1 mL of SSM. The culture dish is then placed in a 25 °C incubator for 24 h. (B) 72-well mini tray that holds brain explants during immunostaining. Brains are washed and solutions transferred using a P20 micropipette set to 10 μL.
Divulgaciones
The authors have nothing to disclose.
Materials
| 10 µL Pipette tips | Denville Sci | P2102 | |
| 1000 µL Pipette tips | Denville Sci | P2103-N | |
| 1000 µL Pipettor | Gilson | P1000 | |
| 24-well multiwell culture plates | Fisher Scientific | 50-197-4477 | |
| 35 mm Petri dishes | Fisher Scientific | 08-757-100A | Grape Plate Ingredients |
| Dissecting microscope | Zeiss | Stemi 2000 | |
| Ethanol 200 proof (100%), Decon Labs, 1 gallon bottle | Fisher Scientific | 2701 | Used to wash off the larvae before the 24 hr hold in culture medium |
| Fetal Bovine Serum (10%) | Sigma | F4135-100ML | Supplement for cell culture media. |
| Fine forceps for dissection | Fine Science Tools | 11295-20 | Forcepts used in disections. They work best when sharpened. |
| Glass Dissection Dish (3 well) | These are no longer available | ||
| Glutathione | Sigma | G6013 | Provides oxidative protection during cell culture. |
| Incubator | Thermo Fisher Scientific | Ensures that the temperature, humidity, and light exposure is exactly the same throughout experiment. | |
| Insulin | Sigma | I0516 | Independant variable of the experiment |
| Laminar flow hood | For aliquoting culture media | ||
| L-Glutamine | Sigma | G7513 | Provides support during cell culture |
| Pen-Strep | Sigma | P4458-100ml | Antibiodics used to prevent bacterial contamination of cells during culture. |
| Phosphate Buffer, pH7.4 | Made in house | Made in house | Solvent used to wash the brains after fixing and staining steps |
| Pick | Fine Science Tools | 10140-01 | Used to pick larvae off of the grape plate |
| Schneiders Culture Medium | Life Tech | 21720024 | Contains nutrients that help the cells grow and proliferate |
| Sterile Water | Autoclave Milli-Q water made in house | Needed for Solutions |

