A Method for the Isolation of Neurovascular Units from Rat Brain
Abstract
Source: Brzica, H., et al,. A simple and reproducible method to prepare membrane samples from freshly isolated rat brain microvessels. J. Vis. Exp. (2018).
This video demonstrates the process of isolating brain microvessels from euthanized rats. It includes the dissection of the brain, cleaning of the tissue, and mechanical digestion, followed by repeated centrifugation to isolate the pure fraction of microvessels.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines. The procedural flow for the protocol is depicted in Figure 1.
1. Set-up for the Procedure
- Prepare the brain microvessel buffer (BMB). Start by weighing 54.66 g D-mannitol, 1.90 g EGTA (Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid), and 1.46 g 2-amino-2-(hydroxymethyl)-1,3-propanediol (i.e., Tris) base into a clean beaker. Add 1.0 L of deionized water. Mix the components of the BMB buffer using a magnetic stirrer. Once the solution has been thoroughly mixed, adjust the pH to 7.4 using 1.0 M HCl.
- Prepare a 26% dextran (MW 75,000) solution (w/v) prior to anesthetizing animals. Follow the following steps to prepare the dextran solution.
NOTE: It is critically important to prepare this solution ahead of time because dextran can take approximately 1.5-2 h to dissolve in an aqueous buffer.- Weigh out the powdered dextran (26 g per 100 mL buffer) into a clean beaker.
- Measure out the appropriate volume of BMB and dispense it into a separate, clean beaker.
- Slowly pour BMB into the beaker containing powdered dextran. During the pouring of BMB, manually mix the powdered dextran using a glass stir rod.
- Adjust the pH to 7.4 with 1.0 M HCl immediately prior to use.
NOTE: A typical isolation of brain microvessels from 12 individual Sprague-Dawley rats (male or female; 3 months of age; 200-250 g each) requires 200 mL of dextran solution (i.e., 52 g dextran in 200 mL of BMB).
2. Extraction of Brain Tissue from Sprague-Dawley Rats
- Following the desired experimental treatment, anesthetize Sprague-Dawley rats using ketamine (50 mg/kg; 2.5 mL/kg i.p.) and xylazine (10 mg/mL; 2.5 mL/kg I,p.). Dilute ketamine and xylazine in 0.9% saline to final concentrations of 20 mg/mL and 4.0 mg/mL, respectively. Euthanize animals by decapitation using a sharpened guillotine in accordance with IACUC (Institutional Animal Care & Use Committee) guidelines. Use the same procedure for both male and female Sprague-Dawley rats.
- Resect the skin from the rat skull by making a single transverse cut using surgical scissors.
- Using rongeur, carefully remove the skull plate and expose the brain.
- Remove the brain using a spatula. Detach the cerebrum and place the isolated brain tissue into a 50 mL conical tube containing 5 mL of BMB. Add 1.0 μL of protease inhibitor cocktail per 1.0 mL of BMB buffer immediately prior to use.
3. Brain Processing
- Transfer the brain tissue from the 50 mL conical tube to a clean petri dish.
- Using forceps, gently roll the brain on 12.5 cm diameter filter paper to remove the outer meninges, which are loosely adhered to the cerebral cortex. Gently press the brain tissue against filter paper and roll the tissue again. Turn the filter paper frequently during this step.
- Separate the choroid plexus from the cerebral hemispheres using forceps.
NOTE: The choroid plexus appears as a clear membranous tissue localized to the surface of the cerebral ventricles. - Gently flatten the brain tissue and remove the remaining meninges and olfactory bulbs using forceps.
- Place the cortical brain tissue into a chilled glass mortar. Add 5.0 mL of BMB containing protease inhibitor cocktail to the mortar.
- Using an overhead power homogenizer, homogenize the brain tissue using 15 up and down strokes at 3,700 rpm. Perform homogenization using a 10 mL mortar and pestle tissue grinder. Between homogenization of each individual sample, clean the pestle using 70% ethanol.
NOTE: Homogenization strokes should be consistent in terms of pace and magnitude. - Pour the homogenate into labeled centrifuge tubes.
4. Centrifugation Steps
- Add 8.0 mL of 26% dextran solution to each labeled centrifuge tube containing brain homogenate.
- Invert the tube twice and then thoroughly vortex the sample. Conduct the vortexing of each sample using multiple angles to ensure a thorough mixing of brain homogenate solution with 26% dextran solution.
- Centrifuge samples at 5,000 x g for 15 min at 4 °C.
NOTE: For some analyses, it is useful to compare brain microvessels with brain parenchyma. Should the assessment of protein expression in brain parenchyma be required, the supernatant from this centrifugation step (i.e., brain parenchymal fraction) can be collected and stored at -80 °C for future use. - Remove the supernatant using a vacuum aspirator and a glass Pasteur pipette.
NOTE: Caution must be taken not to disturb the pellet. Otherwise, the quantity of the brain microvessels collected will be reduced, which will significantly decrease the protein yield from this procedure. - Resuspend the pellet in 5.0 mL of BMB-containing protease inhibitor cocktail (i.e., 1.0 μL protease inhibitor cocktail per 1.0 mL of BMB buffer). Vortex the pellet to ensure thorough mixing.
- Add 8.0 mL of 26% dextran to each centrifuge tube and vortex as described in steps 4.1-4.2 of this protocol.
- Centrifuge samples at 5,000 x g for 15 min at 4 °C.
- Using a vacuum flask and glass pipette, aspirate supernatant and ensure that the pellet containing the brain microvessel is not disrupted.
NOTE: Excess material that has adhered to the tube wall does not contain microvessels and should be carefully cleaned and removed. - Repeat steps 4.5 through 4.8 an additional two times.
- Once 4 dextran centrifugation steps have been completed, add 5.0 mL of BMB to each pellet and vortex to resuspend the sample.
NOTE: Following completion of step 4.10, whole microvessels can be collected for analysis of protein localization. This can be accomplished by taking a 50 μL aliquot of re-suspended microvessel pellet and smearing it on a glass microscope slide. Microvessels are then heat-fixed at 95 °C for 10 min on a heating block, followed by fixation in ice-cold ethanol for an additional 10 min. Slides can then be stored at 4 °C until required for imaging studies.
Representative Results
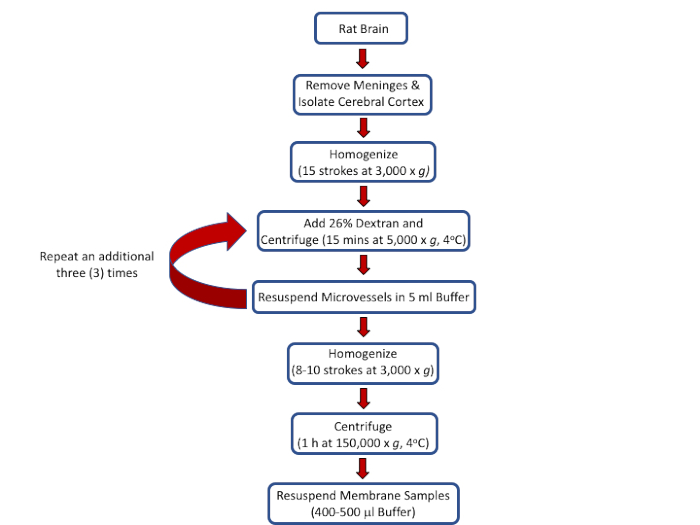
Figure 1: Outline of procedures and protocols to isolate rat brain microvessels and to prepare membrane samples
Divulgaciones
The authors have nothing to disclose.
Materials
| Protease Inhibitor Cocktail | Sigma-Aldrich | #P8340 | Component of brain microvessel buffer |
| D-mannitol | Sigma-Aldrich | #M4125 | Component of brain microvessel buffer |
| EGTA | Sigma-Aldrich | #E3889 | Component of brain microvessel buffer |
| Trizma Base | Sigma-Aldrich | #T1503 | Component of brain microvessel buffer |
| Dextran (MW 75,000) | Spectrum Chemical Mftg Corp | #DE125 | Dextran used in centrifugation steps to separate microvessels from brain parenchyma |
| Zetamine | MWI Animal Health | #501072 | General anesthetic |
| Xylazine | Western Medical Supply | #5530 | General anesthetic |
| 0.9% saline solution | Western Medical Supply | N/A | General anesthetic diluent |
| Filter Paper (12.5 cm diameter) | VWR | #28320-100 | Used for removal of meninges from brain tissue |
| Centrifuge Tubes | Sarstedt | #60.540.386 | Disposable tubes used for dextran centrifugation steps |
| Pierce™ Coomassie Plus (Bradford) Assay | ThermoFisher Scientific | #23236 | Measurement of protein concentration in membrane preparations |
| Wheaton Overhead Power Homogenizer | DWK Life Sciences | #903475 | Required for homogenization of samples |
| 10.0ml glass mortar and pestle tissue grinder | DWK Life Sciences | #358039 | Required for homogenization of samples |
| Hydrochloric Acid | Sigma-Aldrich | #H1758 | Required for pH adjustment of buffers |
| Standard Forceps | Fine Science Tools | #91100-12 | Used for dissection of brain tissue |
| Friedman-Pearson Rongeurs | Fine Science Tools | #16020-14 | Used for opening skull to isolate brain |
| 50 ml conical centrifuge tubes | ThermoFisher Scientific | #352070 | Used for collection of brain tissue following isolation |
| Glass Pasteur Pipets | ThermoFisher Scientific | #13-678-20C | Used for aspiration of cellular debris following dextran spins |
| Ethanol, anhydrous | Sigma-Aldrich | #459836 | Used for cleaning tissue grinder; diluted to 70% with distilled water |
| ketamine | |||
| Ice bucket |

