Purification of CD31+ Endothelial Precursor Cells Using Magnetic-Activated Cell Sorting
Abstract
Source: Matsuo, K., et al. Differentiation of Human Induced Pluripotent Stem Cells to Brain Microvascular Endothelial Cell-Like Cells with a Mature Immune Phenotype. J. Vis. Exp. (2023).
This video demonstrates a technique for purifying CD31+ endothelial progenitor cells (EPCs) from a culture using magnetic-activated cell sorting (MACS). The cell suspension is treated with a fluorophore-conjugated antibody that binds to CD31 on the EPCs. Completing the labeling of CD31+ EPCs involves adding an antibody complex, which binds to the fluorophores, and a polymer-coated magnetic nanoparticle, which binds to the antibody complex. The labeled cells are then purified using a magnetic field, discarding any CD31– cells.
Protocol
The human induced pluripotent stem cell (hiPSC) line, HPS1006, was provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT/ AMED, Japan.
1. Induction of hiPSC differentiation into endothelial progenitor cells (EPCs)
- Extracellular matrix (ECM)-coated plates and reagents
- Prepare basement membrane matrix-coated 12-well plates by aliquoting 2.5 mg of matrix gel into 50 mL centrifuge tubes for storage at -20 °C for up to 6 months. Add 30 mL of cold Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F12), which has been kept in a refrigerator (4 °C), into the tube. Mix gently by pipetting until the gel has thawed and then add 500 µL of the solution to each well of the 12-well plate. Place the plate in an incubator (37 °C, 5% CO2) for at least 1 h.
NOTE: The basement membrane matrix gel is temperature-sensitive and should be handled according to the manufacturer's instructions for aliquoting and plate coating. The concentration of extracellular matrix proteins can vary between batches. To ensure accuracy, the exact concentration should be referred to on the quality certificate sheet for the specific batch, using the lot number. For instance, if the exact concentration is 10.0 mg/mL, use 250 µL of gel for a total of 2.5 mg. - Prepare rho-kinase (ROCK) inhibitor stock solution by dissolving the ROCK inhibitor in sterile water to a concentration of a 10 mM (Table 1). Aliquot the stock solution in 100-200 µL volumes and store at -20 °C to avoid freeze-thaw cycles.
- Make a 100 mg/mL stock solution of L-ascorbic acid by dissolving 5 g of L-ascorbic acid in 50 mL of sterile water and store at -20 °C (Table 1). Add 6.25 mL of glutamine and 305 µL of L-ascorbic acid stock solution into 500 mL of advanced DMEM/F12 to make an LaSR medium (Table 1). Store at 2-8 °C for up to 2 weeks.
- Prepare CHIR99021 solution by dissolving CHIR99021 in undiluted dimethyl sulfoxide (DMSO) to a final concentration of 10 mM (Table 1). Aliquot the solution into 100-200 µL volumes to avoid freeze-thaw cycles and store at -20 °C for up to 1 year. Store working aliquots of the stock solution at 4 °C for up to 1 month.
- Prepare DMEM/F12-10 medium by adding 50 mL of heat-inactivated fetal bovine serum to 450 mL of DMEM/F12. Store the medium at 2-8 °C for up to 1 month (Table 1).
- Prepare flow buffer-1 by adding 33.3 mL of 7.5% bovine serum albumin (BSA) to 467 mL of Dulbecco's phosphate-buffered saline (PBS) (Table 1). Store at 2-8 °C for up to 6 months.
- Prepare basement membrane matrix-coated 12-well plates by aliquoting 2.5 mg of matrix gel into 50 mL centrifuge tubes for storage at -20 °C for up to 6 months. Add 30 mL of cold Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F12), which has been kept in a refrigerator (4 °C), into the tube. Mix gently by pipetting until the gel has thawed and then add 500 µL of the solution to each well of the 12-well plate. Place the plate in an incubator (37 °C, 5% CO2) for at least 1 h.
- Seeding of singularized hiPSCs and expansion for EPC differentiation (Day -3 to Day -1)
- Begin differentiation when the hiPSC colonies in a 6- well plate show no spontaneous differentiation and have the appropriate density for passaging, typically around 80% confluency (2.5-3.5 x 106 cells). Carefully monitor spontaneously differentiated cells under the microscope to ensure whether multiple passages are required to eliminate non differentiated cells. Refer to the note provided below step 1.4.15 for information regarding cell culture medium and extracellular matrix.
- Aspirate the medium and add 1 mL of dissociation reagent to the wells and incubate for 5-7 min at 37 °C. Dissociate and singularize the cells by pipetting the dissociation reagent solution gently over the surfaces of the wells two or three times.
- Transfer the detached cells into a 15 mL tube containing 4 mL of hiPSC maintenance medium and resuspend the cells thoroughly. Reserve a 10 µL aliquot for cell counting.
- Pellet the cells by centrifuging for 5 min at 200 x g at 20-25 °C. Count the cells and calculate the required volume to achieve an appropriate density of hiPSCs (75-400 x 103 per well) in a basement membrane matrix-coated 12-well plate (step 1.1.1).
- Aspirate the coating solution from the wells and add 1 mL of hiPSC medium containing 10 µM ROCK inhibitor into each well (1:1,000 dilution). After centrifuging, aspirate the supernatant and dissociate the pellet in 1 mL of hiPSC medium.
- Add the required volume of hiPSC, determined in step 1.2.4, to each well of the 12-well plate. Two to four 12-well plates may be sufficient for differentiating a hiPSC clone. Refer to step 1.2.4 to determine the number of plates required for seeding cells.
- Place the plate in an incubator (37 °C, 5% CO2). Evenly distribute the cells by gently sliding the plate back and forth and then side to side in the incubator.
- On the following day (i.e., Day -2), exchange the medium with 2 mL of hiPSC maintenance medium lacking ROCK inhibitor. On the next day (Day -1), exchange the medium with 2 mL of fresh hiPSC maintenance medium.
- Induction of EPCs with the glycogen synthase kinase 3 (GSK-3) inhibitor CHIR99021 (Day 0 to Day 5)
- On Day 0, replace the hiPSC maintenance medium in each well with 2 mL of LaSR medium containing 8 µM CHIR99021.
- On Day 1, aspirate the medium and add 2 mL of fresh LaSR medium containing 8 µM CHIR99021.
- On Days 2, 3, and 4, replace the medium with 2 mL of fresh LaSR medium lacking CHIR99021.
- Magnetic activated cell sorting (MACS) to purify CD31+ EPCs (Day 5)
- On Day 5, aspirate the medium and then add 1 mL of dissociation reagent to each well, before incubating for 6-8 min at 37 °C.
- Dissociate and singularize the cells with a micropipette and pass through a 40 µm cell strainer to filter the suspension into a 50 mL tube containing 10 mL of DMEM/F12-10 medium. Filter the cell suspension collected from more than two 12-well plates into at least two 50 mL tubes.
- Stop the digestion reaction by adding DMEM/F12-10 medium (up to 50 mL). Pipette thoroughly and reserve 10 µL for counting cells. Pellet the cells by centrifuging for 5 min at 200 x g at 20-25 °C.
- After removing the supernatant, add 10 mL of DMEM/F12-10 medium and transfer the cell suspension into fresh 15 mL tubes. Pellet the cells by centrifuging for 5 min at 200 x g at 20-25 °C.
- Aspirate the supernatant and resuspend into flow buffer-1 at a density of 1.0 x 107 cells per 100 µL of buffer.
- Add Fc receptor (FcR) blocking reagent at a ratio of 1:100 and incubate for 5 min before adding the fluorescein isothiocyanate (FITC)-labeled CD31 antibody diluted 1:200. Incubate the suspension for 30 min in the dark at 20-25 °C.
- Add 10 mL of flow buffer-1, reserving 10 µL of the suspension for flow cytometry analysis to determine the fraction of CD31+ cells (Figure 1).
- Pellet the cells by centrifuging at 200 x g for 5 min at 20-25 °C. Then, remove the supernatant and resuspend to a density of 1.0 x 107 cells per 100 µL of flow buffer-1 solution. Add the FITC selection cocktail (5 µL per 100 µL of cell suspension). Mix thoroughly by pipetting and incubate in the dark for 15 min at 20-25 °C.
- Add 5 µL of magnetic nanoparticles per 100 µL of cell suspension, pipette well, and incubate in the dark for 10 min at 20-25 °C.
- Transfer the cell suspension to a 5 mL flow cytometry tube and add flow buffer-1 to achieve a total volume of 2.5 mL. Place the flow cytometry tube in the magnet for 5 min.
- In a continuous motion, invert the magnet and decant the cell suspension containing cells that were not labeled with the FITC-CD31 antibody. Maintain the magnet and tube in the inverted position for 2-3 s and then remove the remaining liquid. Aspirate any droplets on the tube edge before returning the tube to an upright position.
- Pick up the flow cytometry tube from the magnet and add 2.5 mL of flow buffer-1 to wash the remaining CD31+ cells. Resuspend the cells by gently pipetting the cells up and down two or three times. Place the flow tube into the magnet for 5 min.
- Repeat steps 1.4.11-1.4.12 three times and then step 1.4.11 once more for a total of four washes.
- Remove the flow tube from the magnet and add the indicated amount of a suitable medium (e.g., human endothelial serum-free medium [hECSR] for extended EC culture or freezing medium for freezing) to the tube to resuspend the purified CD31+ cells. Reserve two 10 µL aliquots of the suspension, one for cell counting and the second to carry out flow cytometry analysis to assess the purity of CD31+ cells in post-MACS samples (Figure 1). If a flow cytometer is not available immediately, store the aliquot at 4 °C until analysis.
- Freeze the CD31+ EPCs at this point.
NOTE: Vitronectin-coated 12-well plates and the more stable hiPSC maintenance medium (mTeSR plus) can be used in place of basement membrane matrix-coated plates and hiPSC maintenance medium (mTeSR1). To prepare vitronectin-coated 12-well plates, dilute vitronectin with dilution buffer to a final concentration of 10 µg/mL and then transfer 500 µL of the diluted solution to each well of the 12-well plates. Leave the plates at 20-25 °C for at least 1 h. The seeding density of singularized hiPSCs is comparable to that used for basement membrane matrix coated plates. Changing the culture medium or the matrix composition may impact the proliferation and spontaneous differentiation of hiPSCs, which typically require 1-2 weeks to adapt to new culture conditions. If the more stable hiPSC maintenance medium is used for hiPSC maintenance, this medium can be used for EPC differentiation instead of the hiPSC maintenance medium. In this case, the more stable hiPSC maintenance medium should be changed at Day -2 to remove ROCK inhibitor, and the exchange on Day -1 can be skipped.
Representative Results
Table 1: Details of specific reagents for the assays. The name and exact amount of ingredients for each specific reagent are described.
| Specific reagent | ingredient | Volume |
| Acetic acid, 0.5 mg/mL solution | Acetic acid | 119 μL |
| Water, sterile, cell culture | 250 mL | |
| Blocking buffer | Skim milk | 5 g |
| Tris-Buffered Saline 1x | 100 mL | |
| Triton X-100 | 300 μL | |
| NaN3, 10% solution in water | 400 μL | |
| CHIR99021 stock solution | CHIR99021 | 10 mg |
| DMSO, sterile | 2.1490 mL | |
| DMEM/F12-10 medium | DMEM-F12 | 450 mL |
| Fetal Bovine Serum | 50 mL | |
| Flow buffer-1 | dPBS | 467 mL |
| BSA, 7.5% in dPBS | 33.3 mL | |
| Freezing medium | hESFM | 30 mL |
| Fetal Bovine Serum | 15 mL | |
| DMSO | 5 mL | |
| ROCK inhibitor Y-27632 | 25 μL | |
| hECSR medium | hESFM | 98 mL |
| FGF2 | 20 μL | |
| B-27 supplement (50x) | 2 mL | |
| Human fibroblast growth factor 2 (bFGF/FGF2) stock solution | FGF2 | 500 μg |
| dPBS | 5 mL | |
| BSA, 7.5% in dPBS | 66.7 μL | |
| L-ascorbic acid stock solution | L-Ascorbic acid | 5 g |
| Water, sterile, cell culture | 50 mL | |
| LaSR medium | Advanced DMEM/F12 | 500 mL |
| GlutaMAX Supplement | 6.25 mL | |
| L-Ascorbic acid | 305 μL | |
| ROCK inhibitor (Y-27632) stock solution | ROCK inhibitor Y-27632 | 10 mg |
| Water, sterile, cell culture | 2.9561 mL | |
| Tris-Buffered Saline (TBS) | Tris Base | 6.05 g |
| NaCl | 8.76 g | |
| distilled water | ~1000 mL |
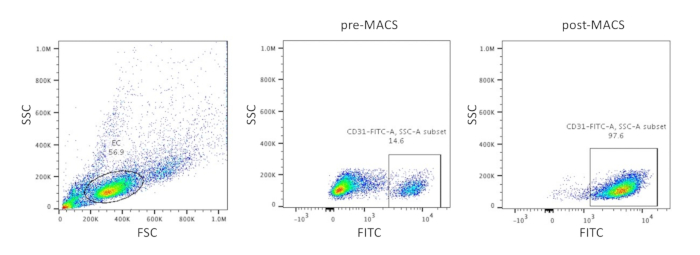
Figure 1: Purification of CD31+ ECs. Dot plots of representative flow cytometry data from scatter gating of ECs and FITClabeled CD31 staining of cell populations before (step 1.4.7) and after (step 1.4.14) MACS. MACS improves the purity of CD31+ EPCs in the population. Abbreviations: SSC = side scatter; FSC = forward scatter; FITC = fluorescein isothiocyanate; MACS = magnetic activated cell sorting.
Divulgaciones
The authors have nothing to disclose.
Materials
| 0.22 mm Syringe filter | TPP | 99722 | |
| 15 mL Centrifuge tube | Falcon | 352196 | |
| 40 μm Falcon cell strainer | Falcon | 352340 | |
| 5 mL Round-bottom tube | SPL | 40005 | |
| 50 mL Centrifuge tube | Falcon | 352070 | |
| 96-Well plate, round bottom | SPL | 34096 | |
| Accutase | Sigma-Aldrich | A6964-500ml | |
| Acetic acid | Sigma-Aldrich | 695092 | |
| Advanced DMEM/F12 | Life Technologies | 12634 | |
| All-in-One Fluorescence Microscope | Keyence | BZ-X810 | |
| B-27 Supplement (503), serum free | Thermo Fischer Scientific | 17504044 | |
| Bovine serum albumin (BSA), 7.5% in dPBS | Sigma-Aldrich | A8412 | |
| CellAdhere Dilution Buffer | STEMCELL Technologies | ST-07183 | |
| CHIR99021 | Selleck Chemicals | S1263 | |
| Collagen IV from human placenta | Sigma-Aldrich | C5533 | |
| Corning tissue culture plates (12-well) | Corning | 3512 | |
| Corning tissue culture plates (6-well) | Corning | 3506 | |
| Cryo tube innercap 2.0 mL | Watson | 1396-201S | |
| Dimethylsulfoxide (DMSO), sterile | Sigma-Aldrich | D2650 | |
| DMEM (13), [+] 4.5 g/L D-glucose, [-] L-glutamine, [-] pyruvate | Thermo Fischer Scientific | 31053-028 | |
| Dulbecco's (d) PBS (without calcium,magnesium) | Thermo Fisher | 14190250 | |
| Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM-F12) | Thermo Fischer Scientific | 11320074 | |
| EasySepFITC Positive Selection Kit II | STEMCELL Technologies | 18558 | |
| EasySepMagnet | Stemcell Technologies | 18000 | |
| Ethylenediaminetetraacetic Acid Solution0.02% in DPBS | Sigma | E8008-100ML | |
| Fetal Bovine Serum, qualified | Thermo Fischer Scientific | 10270106 | |
| HEPES buffer solution | Thermo Fischer Scientific | 15630-056 | |
| Human Endothelial Serum Free Medium (hESFM) | Thermo Fischer Scientific | 11111-044 | |
| Human fibroblast growth factor 2 (FGF2) | Tocris | 233-FB-500 | |
| iPS human induced pluripotent stem cells | Riken RBC | HPS1006 | |
| Kanamycin Sulfate (100x) | Thermo Fischer Scientific | 15160-047 | |
| L-Ascorbic acid 2-phosphate sesquimagnesium salt hydrate | Sigma-Aldrich | A8960-5G | |
| L-Glutamine 200 mM (1003) | Thermo Fischer Scientific | 25030-024 | |
| Matrigel, growth factor reduced | Corning | 354230 | |
| MEM NEAA (1003) | Thermo Fischer Scientific | 11140-035 | |
| ROCK inhibitor Y-27632 | Tocris | 1254 | |
| RPMI medium 1640 Tris base | Sigma-Aldrich | 93362 | |
| Triton X-100 | Sigma-Aldrich | X100 |

