Preparing Free-Floating Slice Cultures from an Adult Human Brain
Abstract
Source: Fernandes, A., et al. Short-Term Free-Floating Slice Cultures from the Adult Human Brain. J. Vis. Exp. (2019).
This video demonstrates a technique for preparing free-floating slice cultures from the adult human brain. Thin slices are generated from a brain section using a vibratome. These slices are then suspended in a culture medium supplemented with growth factors to maintain tissue viability.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Sterilization of materials
NOTE: All material and solutions must be sterilized prior to use.
- Sterilize all surgical tools and vibratome slicing material (knife holder, specimen disk, buffer tray) in a dry sterilizing oven for 4 h at 180 °C.
- Sterilize temperature-sensitive material or equipment by UV or gamma irradiation.
- Sterilize media and solutions by autoclavation or filtration through 0.22 µm pore membranes.
2. Preparation of solutions
- Prepare 15-20 mL of transport solution: 50% v/v Hanks' balanced salt solution (HBSS) pH 7.4, 50% v/v basal medium for maintenance of post-natal and adult brain neurons (Table of Materials), supplemented with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 3 mg/mL glucose, and 33 µg/mL gentamicin.
NOTE: Transport solution must be refrigerated and oxygenated (bubbling with carbogen gas) for at least 20 min prior to sample collection. - Prepare 300 mL of slicing solution (HBSS supplemented with 10 mM HEPES and 3 mg/mL glucose) and cool it down in a freezer to the point of initial crystal formation.
- Prepare 20 mL of culture medium: basal medium for maintenance of post-natal and adult brain neurons (Table of Materials) supplemented with 1% L-glutamine derivative (Table of Materials), 2% supplement for neural culture (Table of Materials), 1% penicillin/streptomycin, and 0.25 µg/mL amphotericin B.
3. Setting up the slicing apparatus
NOTE: This protocol is ideally performed with the assistance of a colleague due to the logistics of sample collection in the surgical room.
- In a bucket of salt-added ice, let the slicing solution rest under carbogen mixture bubbling (95% O2, 5% CO2) for at least 20 min prior to use.
- Prepare a block of 3% agarose (approximately 2 cm x 2 cm x 2 cm) and superglue it to the vibratome specimen disk in order to create additional mechanical support to the tissue sample during slicing (Figure 1E).
- Set the vibratome for slicing: section thickness of 200 µm, frequency of vibration of 100 Hz, and speed of slicing between 0.5-1.0 mm/s.
- Lock the vibratome buffer tray to the vibratome base and add ice to keep it refrigerated prior to receiving the slicing solution and the sample, and throughout the slicing procedure.
4. Sample collection
NOTE: In this protocol, human neocortical tissue was collected in the surgical room and transported to the laboratory.
CAUTION: When dealing with human samples, follow the appropriate safety protocols established by the Institution.
- Set up the transport apparatus (Figure 1C) that consists of: a portable gas cylinder with carbogen mixture connected to a pressure/flux valve that controls the gas output connected to a silicon tubing that connects gas output to the transport vessel; a transport vessel, usually a 50 mL conical centrifuge tube with perforated lid for gas input, containing the transport solution; and ice for sample cooling during transport.
- Collect and transport the specimen (Figure 1B) immediately to the lab. Submerse the specimen in cold transport solution (constantly bubbled with carbogen mixture).
5. Slicing
- Transfer the specimen to a Petri dish (100 mm x 20 mm) containing slicing solution and, with fine surgical tools, carefully remove as much as possible of the remaining meninges in the sample (Figure 1D).
- Choose the best specimen orientation for producing slices with the particular characteristics of the experimental design, and with a no. 24 scalpel blade, trim a flat surface to be the base glued to the specimen disk.
- Using a disposable plastic spoon and delicate paintbrushes, collect the fragment from the Petri dish and dry excess solution using filter paper (dry by capillarity and avoid touching the tissue fragment with paper).
- Using superglue, attach the tissue to the vibratome specimen disk until it is firmly adhered to the disk and in contact with the agarose block (Figure 1E).
- Place the vibratome specimen disk (with tissue properly attached) in the vibratome buffer tray filled with slicing solution that must be bubbling during the whole process.
- Lock the knife holder in place with the razor blade firmly fixed.
- The slicing solution must cover both the specimen and the blade, only then start slicing (Figure 1F).
- Cut the specimen into 200 µm slices.
NOTE: Although some vibratomes cut the specimens automatically, the close observation and minor adjustments in slicing speed during the process may help producing better slices. Discard initial irregular slices. - Transfer the slices from the buffer tray to a Petri dish with slicing solution and trim loose edges and excess white mater to a proportion of around 70% cortex/30% white matter.
6. Culture
NOTE: Perform this step in a laminar flow cabinet under sterile environment.
- Add 600 µL of culture medium per well (in a 24 well plate) and incubate for at least 20 min at 36 °C and 5% CO2 prior to plating the slices.
- Plate one slice per well using a paintbrush (Figure 1G).
- If there are any unused wells in the plate, fill them with 400 µL of sterile water.
- Incubate the plate at 36 °C, 5% CO2.
NOTE: First medium replacement must be done in between 8-16 h after plating depending on the size of the slice. - Supplement 10 mL of the previously prepared culture medium with 50 ng/mL brain derived neurotrophic factor (BDNF).
NOTE: During the first 8-16 h, the slices are incubated in 600 µL of medium to avoid nutrient deprivation and acidification, since medium consumption in this phase is accelerated. From the next step, the volume of medium per well is adjusted to 400 µL. - Remove 333 µL of the conditioned medium from each well and add 133 µL of fresh BDNF-supplemented medium.
- Repeat the process of medium replacement every 24 h by replacing one-third of the conditioned medium with fresh BDNF-supplemented medium.
Representative Results
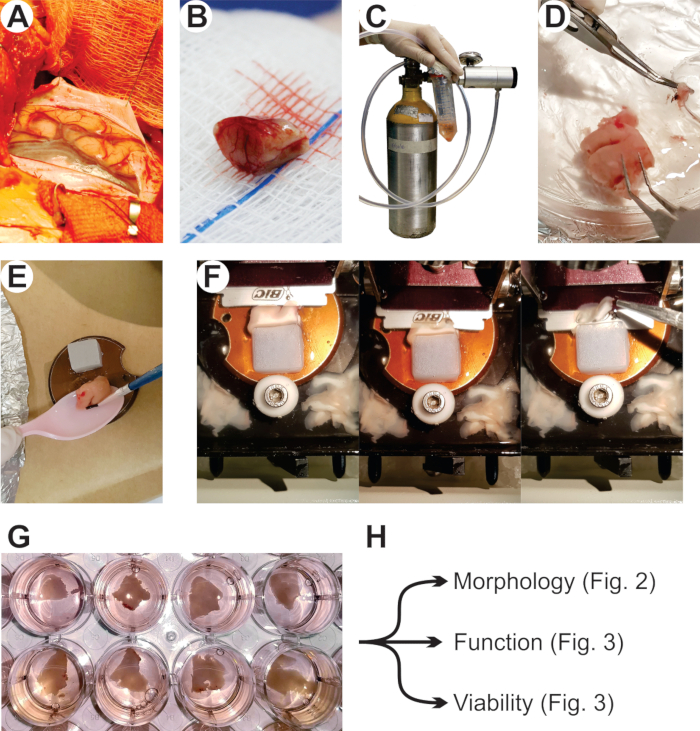
Figure 1: Sample collection, transport, slicing, and culturing of cortical tissue from adult humans. The procedure starts at the surgical room with collection of cortical tissue from temporal lobectomy for the treatment of pharmacoresistant epilepsy (A,B). (C) Tissue fragment (n = 1) is immediately transferred to a tube containing ice-cold oxygenated transport medium (see below). (D) In the lab, meninges are removed using fine ophthalmic tweezers. Excess liquid is dried using filter paper, and the fragment is superglued (E) to the vibratome specimen disk with the white matter facing down and pial surface facing up. (F) Using a commercial shaving razor, the specimen is cut into 200 µm slices that are collected with a delicate paintbrush and transferred back to the Petri dish for further trimming of excess white matter and loose ends (not shown) prior to (G) plating and culturing in a free-floating format. (H) Slices cultures are kept viable for several days and can be used in a variety of experimental protocols.
Divulgaciones
The authors have nothing to disclose.
Materials
| Agarose | Sigma Aldrich | A9539 | |
| Ammonium persulfate | Sigma | A3678-25G | |
| Amphotericin B | Gibco | 15290-018 | |
| B27 | Gibco | 17504-044 | |
| BDNF | Sigma Aldrich | SRP3014 | |
| Bovine Serum Albumin | Sigma Aldrich | A7906 | |
| Glucose | Merck | 108337 | |
| Glutamax | Gibco | 35050-061 | |
| Hank's Balanced Salts | Sigma Aldrich | H1387-10X1L | |
| Hepes | Sigma Aldrich | H4034 | |
| Neurobasal A | Gibco | 10888-022 | |
| PBS Buffer pH 7.2 | Laborclin | 590338 | |
| Penicilin/Streptomicin | Sigma Aldrich | P4333 | |
| Equipment and Material | |||
| Carbogen Mixture | White Martins | 95% O2, 5% CO2 | |
| CO2 incubator | New Brunswick Scientific | CO-24 | Incubation of slices 5% CO2, 36ºC |
| Plastic spoon | Size of a dessert spoon | ||
| Razor Blade | Bic | Chrome Platinum, used in slicing with vibratome | |
| Scalpel Blade | Becton Dickinson (BD) | Number 24 | Used for slicing of tissue; recommended same size or smaller |
| Superglue (Loctite Super Bonder) | Henkel | Composition: Etilcianoacrilato; 2-Propenoic acid; 6,6'-di-terc-butil-2,2'-metilenodi-p-cresol; homopolymer | |
| Vibratome | Leica | 14047235612 – VT1000S |

