Bladder Tumor Organoid Model: A Method for Orthotopic Transplantation of Bladder Organoids into Recipient Murine Bladder
Abstract
Source: Kim, Y. et al. Culture, Manipulation, and Orthotopic Transplantation of Mouse Bladder Tumor Organoids. J. Vis. Exp. (2020)
In this video, we describe the orthotopic transplantation of cultured bladder tumor organoids into the murine bladder to study the role of the organ environment on developing tumors.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Subculture Bladder Tumor Organoids
NOTE: Passage of bladder tumor organoids when they reach 100–150 µm in diameter is recommended.
- Add 500 µL of collagenase/dispase to the organoid medium in a 24-well plate with tumor organoids. Pipette up and down the basement membrane matrix and the medium. Incubate for 20 min at 37 °C and harvest the cells into a 15 mL tube.
NOTE: Examine the organoids isolated from the basement membrane matrix under a microscope. If the organoids are not detached from the basement membrane matrix, increase the incubation time or pipette more times. - Add 5 mL of prewarmed DMEM, centrifuge the tube at 400 x g for 3 min at 4 °C, and aspirate the supernatant.
- Resuspend the pellet using 1 mL of prewarmed 0.25% trypsin-EDTA and 10 µM Y-27632. Incubate for 5 min in a 37 °C water bath. Vigorously pipette the cells up and down and neutralize the trypsin using 5 mL of DMEM with 10% FBS.
- Centrifuge the tube at 400 x g for 3 min at 4 °C and aspirate the supernatant.
- Resuspend the pellet using 1 mL of prewarmed organoid medium and count the number of single tumor cells.
2. Orthotopic Transplantation of Bladder Organoid (Figure 1A)
- Prepare the bladder tumor organoids for orthotopic transplantation
- Before transplantation, culture the bladder tumor organoids for 5–7 days, as described above.
- Add 500 µL of collagenase/dispase to the organoid medium in a 24-well plate with the tumor organoids. Pipette up and down the basement membrane matrix and medium. Incubate for 20 min at 37 °C and collect the cells into a 15 mL tube.
- Add 5 mL of prewarmed DMEM, centrifuge the tube at 400 x g for 3 min at 4 °C, and aspirate the supernatant.
- Resuspend the pellet with 1 mL of DMEM and transfer the solution into a 90 mm Petri dish.
- Under a microscope, pick up the 10–100 tumor organoids by using a P200 micropipette and collect them into a microtube on ice.
- Centrifuge the tube at 400 x g for 3 min at 4 °C and carefully discard the supernatant.
- Maintain the cell pellet on ice until the mice are ready for surgery.
- Submucosal bladder wall transplantation
- Prepare an 8- to 10-week old male nude mouse (CAnN.Cg-Foxn1nu/Crl) at least 1 week before the experiment to allow it to acclimate to a new environment. Inject enrofloxacin (5 mg/kg) subcutaneously 24 h before surgery.
- Clean the bench surface with soap and water. Autoclave the surgical instruments prior to the surgical procedure and perform surgery using sterile instruments.
- Keep the 29 G insulin syringe, pipette tips, and basement membrane matrix on ice. Administer ketoprofen (5 mg/kg) subcutaneously before administration of anesthesia.
- Anesthetize the mouse with 4% isoflurane in an induction chamber. Once general anesthesia is achieved, lay the mouse in a supine position and maintain anesthesia by mask inhalation of 2% vaporized isoflurane.
NOTE: If the anesthetization time is over 30 min, apply eye ointment to both eyes using a cotton swab to avoid corneal drying. - Apply povidone-iodine with sterile gauze and wipe it down with 70% ethanol. Repeat 3x with a new gauze or a cotton swab each time.
- Cover the anus and the surgical field using disposable, sterile surgical drapes.
- Using a dissecting microscope for magnification, make a small transverse incision (smaller than 1.5 cm) in the skin and muscular wall of the lower midline abdomen with sterile surgical scissors. Expose the bladder from the abdominal cavity and support it with saline-soaked cotton swabs.
NOTE: If the bladder is full of urine, gently press the bladder to decompress it slightly. - Resuspend the organoid pellets in 80 µL of organoid medium containing 50% high-concentration basement membrane matrix (Table 1 and Table of Materials).
- Inject the organoid suspension into the anterior aspect of the bladder dome using the 29 G insulin syringe under a dissecting microscope.
- Close the inner layer of the abdominal wall with antibacterial absorbable suture and then close the outer layer with 4-0 nylon suture. Disinfect the surgical site with povidone-iodine and 70% ethanol.
- Allow the mouse to recover under an infrared irradiator 10–15 min. Monitor the mouse until it regains consciousness and motility.
- One day after surgery, check the general condition of the mouse and anastomotic leakage. Administer ketoprofen (5 mg/kg) once daily for 3 days postoperatively and treat enrofloxacin (5 mg/kg) once daily for 10 days postoperatively.
- When the incision site has healed (10–14 days after surgery), remove the sutures. Monitor the growth of the mouse bladder tumor for 2–3 weeks after the tumor organoid injection.
- If bladder tumor growth is observed, euthanize the mouse using carbon dioxide inhalation, and harvest the entire bladder tumor. Wash it using cold DPBS (Figure 1B).
- To analyze the bladder tumor histology, stain the paraffin-embedded section of the tissue using hematoxylin and eosin (H&E) staining (Figure 1B).
| Mouse bladder tumor organoid medium | |
| Advanced DMEM/F-12 (Basic medium) | 10 mM HEPES (pH 7.4) |
| 10 mM Nicotinamide | 0.5x Serum-free supplement |
| 2 mM L-alanyl-L-glutamine dipeptide | 1% Penicillin/Streptomycin |
| 1 mM N-acetyl-L-cysteine | 50 ng/mL Murine epidermal growth factor |
| 1 µM A 83-01 | |
Table 1: Composition of bladder tumor organoid medium.
Representative Results
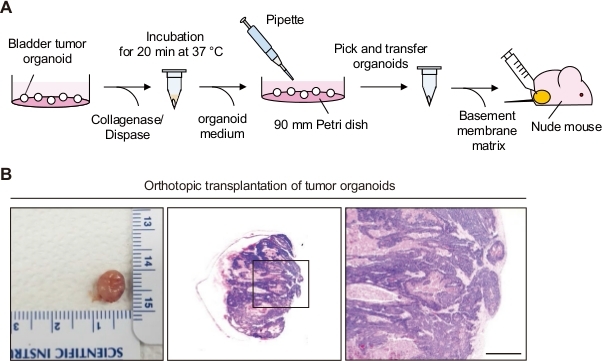
Figure 1: Orthotopic transplantation of bladder tumor organoids. (A) Schematic diagram of orthotopic transplantation of bladder tumor organoids to a nude mouse. (B) Representative images of bladders and H&E stained sections from mice orthotopically transplanted with bladder tumor organoids. Magnified views of the boxed regions in the middle panels are shown in the left panels. Scale bar = 500 µm.
Divulgaciones
The authors have nothing to disclose.
Materials
| 15 mL conical tube | SPL | 50015 | |
| 24-well plate | Corning | 3526 | |
| 29 G 1/2 insulin syringe | SHINA | B299473538 | |
| 50 mL conical tube | SPL | 50050 | |
| A8301 | Tocris | 2939 | Stock concentration: 25 mM |
| Absolute ethanol | Daejung | 4023-2304 | |
| Absorbable suture | Henry Schein | 039010 | |
| Advanced DMEM/F-12 | Thermo | 12634028 | |
| CAnN.Cg-Foxn1nu/Crl (nude mouse) | Charles River | 194 | |
| Collagenase type I | Thermo | 17100017 | Stock concentration: 20 mg/mL |
| Collagenase type II | Thermo | 17100015 | Stock concentration: 20 mg/mL |
| Collagenase/dispase | Sigma | 10269638001 | Stock concentration: 1 mg/mL |
| DMEM (Dulbecco's modified minimum essential media) | Gibco | 11965-118 | |
| DMSO (Dimethyl sulfoxide) | Sigma | D8418 | |
| DPBS (Dulbecco's phosphate-buffered saline) | Welgene | LB 001-02 | |
| Matrigel growth factor reduced (GFR) Growth Factor Reduced (GFR) | Corning | 354230 | Use for organoid culture in plate |
| Matrigel high concentration (HC) | Corning | 354248 | Use for organoid transplantation |
| 1.5 mL microtube | Axygen | MCT-150-C | |
| Razor blade | |||
| Saline buffer | JW Pharmaceutical | ||
| Trypsin-EDTA (0.25%) | Gibco | 25200072 | |
| Ultracentrifugation tube | Beckman Coulter | 331372 | |
| Y-27632 dihydrochloride | Abmole | M1817 | Stock concentration: 10 mM |

