Extracting Intact Kidney from Mouse: A Technique to Obtain Kidney Without Renal Capsule from Murine Models
Abstract
Source: Grainger, N. et al. Isolating and Imaging Live, Intact Pacemaker Regions of Mouse Renal Pelvis by Vibratome Sectioning. J. Vis. Exp. (2021)
In this video, we describe the procedure for kidney extraction from the retroperitoneal space of the mouse. The extracted kidney can further be microdissected to obtain an intact kidney without a renal capsule and the surrounding fat layer.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Kidney Dissection
- Anaesthetize mice by inhalation of 3–4% isoflurane in a ventilated hood. Confirm the induction of deep anesthesia by loss of toe and/or tail pinch reflex and then euthanize the mice by cervical dislocation.
- Apply 70% ethanol to the chest to dampen the fur. Using external dissection scissors, open the abdominal cavity via a longitudinal incision, with scissor blades angled away from the animal to prevent damage to the internal organs.
- Using internal tissue forceps and internal dissection scissors, pinch the intestines and lift them away from the abdominal wall. Whilst lifting the intestines, cut the underside of the intestines free from the body at the proximal duodenum and distal colon to gain access to the retroperitoneal space containing the kidneys.
- Once the kidneys are exposed, extract them individually. Gently pinch and lift the distal end of the ureter (~4mm away from the kidney) with tissue forceps. Using the dissection scissors, cut underneath the pinched ureter towards the kidney. Continue to cut underneath the kidney until it is liberated from the surrounding connective tissue.
NOTE: To maximize tissue integrity and cutting consistency during vibratome sectioning, the kidney must be as intact as possible. To ensure this, avoid pinching or cutting of the kidney with forceps and dissection scissors. - Place the kidney with attached ureter in an ice-cold KRB solution. Repeat the extraction steps with the contralateral kidney to remove from mouse's body. Maintain the kidneys in KRB solution on ice.
NOTE: Immediately proceed to the next section of the protocol to preserve PKJ tissue viability. Because of its anatomical location deep in the kidney parenchyma, the PKJ is deprived of contact with KRB solution.
2. Prepare the Kidney Without Renal Capsule for Vibratome Sectioning
- Transfer the kidney to a silicon elastomer-coated dissection dish (60 mm x 15 mm), and fill it with ice-cold KRB solution until the kidney is completely submerged.
- Under a dissecting microscope, anchor the kidney to the base of the dish by inserting minutien pins into the proximal ureter and through the thin anterior renal capsule or surrounding adipose tissue.
NOTE: Take care not to puncture the kidney parenchyma tissue. - Use fine spring scissors and internal forceps to remove the adipose tissue from the base of the kidney to expose the distal RP and proximal ureter.
- Remove the proximal ureter and a portion of the distal RP from the base of RP using fine spring scissors.
NOTE: Take care not to cut the surrounding kidney parenchyma. The black dashed line in Figure 1A indicates the approximate position of this cut. This cut creates a flat base on the kidney for more uniform tissue sectioning. When dissecting the kidney, one must be aware of the anatomical position of the PKJ region. Figure 1B shows that the intact kidney can be cut along a sagittal plane to expose the medulla, papilla (distal medulla where collecting ducts converge), and proximal and distal RP. If the papilla were to be exposed completely, as in Figure 1C, the PKJ and proximal RP (prox RP) can be visualized. However, this should not be done for the vibratome technique; this description is to orientate the reader to the PKJ location generally, emphasized in the anatomical difference shown in the transmitted light images of the PKJ region and mid-RP region in Figure 1D, E. - Pierce the outer renal capsule with fine-tip forceps, angling the tips away from the kidney body. Using forceps with each hand, pinch the loose ends of the capsule and peel them apart. Continue to peel back the remaining renal capsule membrane until it is removed entirely.
NOTE: The renal capsule is a tough, fibrous layer that surrounds the kidney. It must be removed prior to vibratome sectioning for optimal cutting.
Representative Results
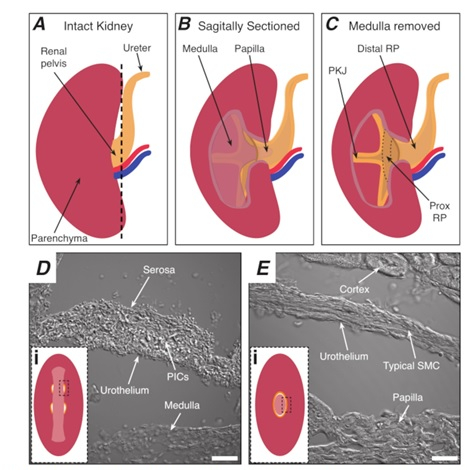
Figure 1: Basic kidney anatomy and location of PKJ pacemaker region. (A) Diagram of the intact kidney showing the orientation of the RP and ureter. The renal artery and renal vein are displayed in red and blue, respectively. (B) The intact kidney can be cut along a sagittal plane to expose the inner aspect of the kidney, including the medulla, papilla (distal medulla where collecting ducts converge), and proximal and distal RP. (C) The medulla and papilla can be excised to completely expose the PKJ and prox RP. (D and E) represent transmitted light images from the PKJ pacemaker region and distal RP, respectively. Sequential sectioning towards the distal end of the pelvis results in the semicircles of muscle in the PKJ region (Di) combining into one, thicker muscular ring (Ei) that encapsulates the entire papilla. Black, dashed rectangles in Di and Ei show approximate areas in coronal kidney sections where transmitted light images were acquired. Orientation of images D and E are 90° anti-clockwise to respective insets (Di and Ei). Scale bars in D and E = 20 µm. Abbreviations: RP = renal pelvis; prox RP = proximal renal pelvis; PKJ = pelvic-kidney junction; PICs = platelet-derived growth factor receptor-alpha-positive interstitial cells; SMC = smooth muscle cell.
Divulgaciones
The authors have nothing to disclose.
Materials
| 60 mm x 15 mm Petri dish | Sigma Aldrich | P5481 | Kidney sharp dissection dish |
| Absorbent paper | Fisher Scientific | 06-666A | To dry the kidney before applying glue |
| B6;129S-Gt(ROSA)26Sor/J | The Jackson Laboratory | 13148 | GCaMP3 Mice |
| B6;129S-Gt(ROSA)26Sor/J | The Jackson Laboratory | 24105 | GCaMP6f Mice |
| B6.FVB-Tg(Myh11-cre/ERT2)1Soff/J | The Jackson Laboratory | 19079 | smMHC-CRE Mice |
| C57BL/6-Tg(Pdgfra-cre)1Clc/J | The Jackson Laboratory | 13148 | PDGFRa-CRE Mice |
| Ethanol | Phamco-Aaper | SDA 2B-6 | For dissection |
| Extra-fine Bonn Scissors | Fine Science Tools | 14083-08 | Used for internal dissecting scissors |
| Fine scissors | Fine Science Tools | 14060-09 | Used for external dissecting scissors |
| Fine-tip forceps | Fine Science Tools | 11254-20 | Used for fine dissection of kidney |
| Isoflurane | Baxter | NDC 1001936060 | For anesthesia |
| Silicon elastomer | Fisher Scientific | NC9285739 | Sylgard 184 |
| Student Adson Forceps | Fine Science Tools | 91106-12 | For gently holding and moving the kidney |
| Student Dumont Forceps | Fine Science Tools | 91150-20 | Used for internal dissecting forceps |
| Vannas spring scissors | Fine Science Tools | 15000-03 | For sharp dissection and cleanup of isolated kidney |

