Summary
The surgical procedure for induction of unilateral hindlimb ischemia is demonstrated, with confirmation of ischemia by laser Doppler perfusion imaging.
Abstract
In the United States, peripheral arterial disease (PAD) affects about 10 million individuals, and is also prevalent worldwide. Medical therapies for symptomatic relief are limited. Surgical or endovascular interventions are useful for some individuals, but long-term results are often disappointing. As a result, there is a need for developing new therapies to treat PAD. The murine hindlimb ischemia preparation is a model of PAD, and is useful for testing new therapies. When compared to other models of tissue ischemia such as coronary or cerebral artery ligation, femoral artery ligation provides for a simpler model of ischemic tissue. Other advantages of this model are the ease of access to the femoral artery and low mortality rate.
In this video, we demonstrate the methodology for the murine model of unilateral hindimb ischemia. The specific materials and procedures for creating and evaluating the model will be described, including the assessment of limb perfusion by laser Doppler imaging. This protocol can also be utilized for the transplantation and non-invasive tracking of cells, which is demonstrated by Huang et al.1.
Protocol
1. Induction of Unilateral Hindlimb Ischemia
- The surgical tools needed for this operation include: fine pointed forceps, pointed forceps, spring scissors, surgical scissors, needle holder, and retractor. We make our own retractor using a paperclip because it is smaller than commercially available retractors. Sterilize these tools prior to surgery by an autoclave or a hot-bead sterilizer. A cautery tool and sterile fine pointed cotton swabs will also be needed for this surgery. It is recommended that the tools be re-sterilized at the tips as needed during the procedure.
- When the tools are ready, place the mouse into the anesthesia induction chamber containing 1–3% isoflurane in 100% oxygen at a flow rate of 1L/min.
- Leave the mouse in the induction chamber until it is unresponsive to external stimuli. Then remove the animal from the induction chamber. It is recommended to flush the anesthetic from the box prior to opening the lid, to decrease operator exposure to isoflurane.
- Then place the animal in the supine position onto the pre-operating table and connect it to a continuous flow of isoflurane. Using an electric shaver, remove the hair from the hindlimb. Apply hair removal cream to thoroughly remove hair.
- Place the mouse in the supine position over a draped heated pad on the operating table, and connect it to a continuous flow of isoflurane. Extend and secure the hindlimb with a piece of tape. Once the hindlimb is secure, wipe the skin with three alternating betadine and alcohol scrubs. For the remainder of the surgical procedure, use a dissection microscope at 10X or 20X magnification to obtain an enlarged view of the hindlimb region.
- Using fine forceps and surgical scissors, make an incision of the skin, approximately 1 cm long, from the knee towards the medial thigh.
- Using phosphate buffered saline (PBS)-moistened fine pointed cotton swabs, gently brush away subcutaneous fat tissue surrounding the thigh muscle.
- Next, apply the cautery transversely to incise and dissect through the subcutaneous fat tissue to reveal the underlying femoral artery.
- Use a retractor to open the wound and to obtain a better view of the lower extremity vasculature.
- Using fine forceps and a fine pointed cotton swab, gently pierce through the membranous femoral sheath to expose the neurovascular bundle.
- Then, using a clean set of fine forceps and cotton swab, dissect and separate the femoral artery from the femoral vein and nerve at the proximal location near the groin. After the dissection, pass a strand of 7-0 silk suture underneath the proximal end of the femoral artery. Occlude the proximal femoral artery using double knots. Place the tie on the vessel as proximal in the wound as possible in order to leave length for the second tie and an intervening segment that will be transected.
- Separate the femoral artery from the femoral vein at the distal location close to the knee. Pass a strand of 7-0 suture underneath the distal end of the femoral artery proximal to the popliteal artery. Occlude the vessel using double knots.
- Occlude the distal femoral artery with a second set of double knots just proximal to the first set of knots. This second set of sutures will be used for gripping the artery during the transaction procedure.
- Similarly, for gripping purposes, occlude the proximal femoral artery with a second set of double knots just distal to the first set of knots.
- Transect the segment of femoral artery between the distal and proximal knots with a fine pointed cotton swab and a pair of spring scissors. Use caution not to pierce the femoral vein wall.
- Remove the retractor and close the incision using 5-0 Vicryl sutures. These sutures do not need to be removed at a later time, as they dissolve on their own.
- Once the incision is closed, place the animal on top of a draped heated pad in the recovery cage and monitor continuously until awake.
- After animal has recovered for 1 hour, proceed with the laser Doppler blood perfusion step in order to confirm the ischemia induction.
2. Laser Doppler Blood Perfusion
- To begin the laser Doppler perfusion step, place the mouse into the anesthesia induction chamber containing 1–3% isoflurane in 100% oxygen at a flow rate of 1L/min.
- Leave the mouse in the induction chamber until it is unresponsive to external stimuli. Then remove the animal from the induction chamber. It is recommended to flush the anesthetic from the box prior to opening the lid, to decrease operator exposure to isoflurane.
- Place the animal on the pre-operating table connected to continuous flow of isoflurane. Then remove the hair from the hindlimb using an electric shaver followed by hair removal cream as necessary.
- After removing the hair, place the animal on a 37°C heated surface for 5 minutes under continuous flow of isoflurane. Monitor the core temperature to ensure euthermia, as temperature changes will markedly affect perfusion.
- After 5 minutes, place the animal in the supine position on a non- reflective light- absorbing surface such as green-colored cloth, connected to a continuous flow of isoflurane. Extend the hindlimbs.
- Next, turn on the laser Doppler imager and the acquisition software and initialize the software. Specify the size of the field of view and resolution. It is best to keep the field of view and pixel density consistent between animals in order to make future analysis easier.
- Open a new file. Press start to begin acquiring the image data. Generally, the imager automatically detects the distance of the mouse, but if prompted, specify the distance of the animal to the imager. The laser will show the borders of the field of view and then start acquiring data.
- After acquisition is complete, the image will begin to show a range of colors that are indicative of the level of blood perfusion to the legs. The colors can be set to a specific perfusion range to better compare data between animals.
- When the data acquisition is complete, save the file.
- Then return the animal to the recovery cage and monitor the animal continuously until awake.
- To analyze the data, use the threshold adjustment in the analysis software to substract any background noise. A threshold of 0.2 is usually a reasonable value. Select 2 regions of interest, or ROIs, that cover each hindlimb area. A variety of landmarks can be used to standardize the ROI between limbs and animals. Then determine the mean perfusion and variability in the ROI. The perfusion difference and perfusion ratio between the ischemic limb and control limb can then be easily determined.
- This procedure can be repeated to follow changes in hindlimb perfusion over time. At a desired time point, the animal can be euthanized for assessment of tissue function and comparison to perfusion image data.
3. Representative Results:
The anatomy of the hindlimb vasculature is shown in Figure 12. A representative diagram of the hindlimb after femoral artery explantation is shown in Figure 2. To confirm the induction of ischemia to the hindlimb, laser Doppler perfusion image analysis demonstrates a dramatic reduction in blood flow to the ischemic limb, in comparison to the control limb, as shown in Figure 3.
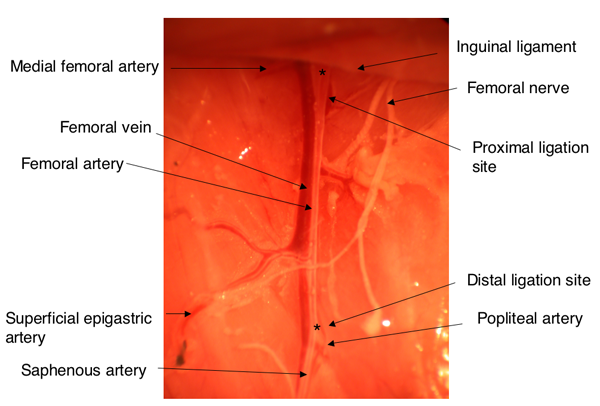
Figure 1. Anatomy of the hindlimb vasculature. Asterisks indicate the locations of ligation for the induction of hindlimb ischemia.
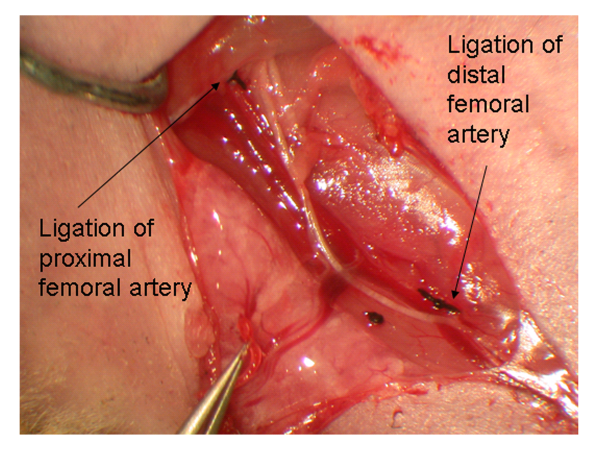
Figure 2. Representative diagram showing the anatomy of the hindlimb after ligation of the femoral artery at the proximal and distal sites after the removal of the femoral artery.
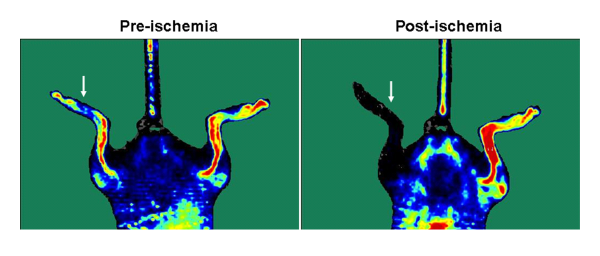
Figure 3. Laser Doppler images showing blood flow before and after the induction of ischemia to the left hindlimb (indicated by arrow).
Discussion
There are some sources of variability to consider while planning and performing hindlimb ischemia models. First, the level of ischemia may vary according to the location of the ligation with respect to that of side branches. Therefore, for consistency of the model, the animals should undergo the same level of arterial ligation. Another source of variability in ischemic recovery is related to the age of the animals, with young animals (6–8 weeks old) having faster and more complete recovery rates than older animals (8–10 months old), as assessed by hemodynamic (i.e. laser Doppler perfusion) or functional (i.e. treadmill testing) measures. For studies in which one is assessing an angiogenic agent, it may be preferable to use older animals, because a greater difference between groups may be observed with a therapeutic intervention. Conversely, for studies in which one is assessing an anti-angiogenic factor, it may be preferable to use younger animals to maximize effect size3.
If performed correctly, there should be minimal complications such as bleeding, infection, or mortality. If bleeding occurs by accidental disruption of the femoral vein or other vessels, moderate pressure with a sterile cotton tipped applicator or gauze should be applied to the site of hemorrhage until the bleeding stops. Animals showing signs of infection should be treated with anti-infective agents. Animals exhibiting significant gangrene may need to beeuthanized. This complication is more common in older animals, and in some strains, such as BALB c mice4. In addition, the hindlimb ischemia model may cause mild to moderate pain. Therefore, analgesics such as buprenorphine or carprofen should be administered as needed for treatment of pain, according to recommendations of the IACUC.
In conclusion, we have demonstrated a simple and reproducible method for studying PAD in a murine model of hindlimb ischemia. The hindlimb ischemia model with laser Doppler imaging analysis is an excellent system for studying vascular pathologies and for assessing therapeutic candidates.
Acknowledgements
The authors thank Andrea Axtell, Satoshi Itoh, MD, Jeff Velotta, MD, Grant Hoyt, Robert C. Robbins, MD, Jin Yu, MD, Tim Doyle, PhD, and the Stanford Small Animal Imaging Core for technical assistance. The authors also thank A.M. Bickford, Inc. for support of veterinary equipment. This research was supported by research grants from the National Institutes of Health (R01 HL-75774, R01 CA098303, R21 HL085743, 1K12 HL087746), the California Tobacco Related Disease Research Program of the University of California (15IT-0257 and 1514RT-0169), and the California Institute for Regenerative Medicine (RS1-00183). N.H. is supported by a fellowship from the American Heart Association.
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Surgical Tools | Tool | Fine Science Tools | ||
| Constix Cotton Swabs | Tool | Contec | SC-4 | |
| Betadine (Povidone-iodine) | Reagent | PDI | ||
| 70% alcohol | Reagent | Kendall | ||
| Phosphate Buffered Saline | Reagent | Invitrogen | ||
| 7-0 silk suture | Tool | Genzyme | ||
| 5-0 vicryl suture | Tool | Ethicon | ||
| Electric shaver | Tool | GE | ||
| Cautery | Tool | Baxter | ||
| Laser Doppler and PeriScan PIM 3 System | Equipment | Perimed |
Referencias
- Huang, N. F., Niiyama, H., De, A., Cooke, J. P., , . Transplantation and non-invasive tracking of embryonic stem cell-derived endothelial cells for treatment of hindlimb ischemia. J Vis Exp. , (2008).
- Cook, M. J. . The anatomy of the laboratory mouse. , (1976).
- Niiyama, H., Kai, H., Yamamoto, T., Shimada, T., Sasaki, K., Murohara, T., Egashira, K., Imaizumi, T. Roles of endogenous monocyte chemoattractant protein-1 in ischemia-induced neovascularization. J. Am. Coll. Cardiol. 44, 661-666 (2004).
- Dokun, A. O., Keum, S., Hazarika, S., Li, Y., Lamonte, G. M., Wheeler, F., Marchuk, D. A., Annex, B. H. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation. 117, 1207-1215 (2008).

