Reconstructing Terrestrial Paleoclimate and Paleoecology with Fossil Leaves Using Digital Leaf Physiognomy and Leaf Mass Per Area
Summary
The protocol presented shows digital measurement and analysis of continuous leaf physiognomic traits on fossil leaves to reconstruct paleoclimate and paleoecology using the digital leaf physiognomy and leaf mass per area reconstruction methods.
Abstract
Climate and environment strongly influence the size, shape, and toothiness (physiognomy) of plants' leaves. These relationships, particularly in woody non-monocotyledonous angiosperms, have been used to develop leaf-based proxies for paleoclimate and paleoecology that have been applied to reconstruct ancient terrestrial ecosystems for the last ~120 million years of Earth's history. Additionally, given that these relationships have been documented in living plants, they are important for understanding aspects of plant evolution and how plants respond to climatic and environmental changes. To conduct these types of analyses on modern and fossil plants, leaf physiognomy must be measured accurately using a reproducible methodology. This protocol describes a computer-based method for measuring and analyzing a variety of leaf physiognomic variables in modern and fossil leaves. This method allows for the measurement of leaf physiognomic traits, in particular variables related to leaf serrations, leaf area, leaf dissection, and linearity that are used in the digital leaf physiognomy proxy for reconstructing paleoclimate, as well as petiole width and leaf area, which are used for reconstructing leaf mass per area, a paleoecological proxy. Because this digital leaf trait measurement method can be applied to fossil and living plants, it is not limited to applications related to reconstructing paleoclimate and paleoecology. It can also be used to explore leaf traits that may be informative for understanding the function of leaf morphology, leaf development, phylogenetic relationships of leaf traits, and plant evolution.
Introduction
Leaves are fundamental production units that facilitate the exchange of energy (e.g., light, heat) and matter (e.g., carbon dioxide, water vapor) between the plant and its surrounding environment1,2. To perform these functions, leaves must mechanically support their own weight against gravity in still and windy air 3,4. Because of these intrinsic links, several aspects of the size, shape, and toothiness of leaves (physiognomy) reflect the details of their function and biomechanics and provide insight into their environment and ecology. Prior work has quantified relationships between leaf physiognomy, climate, and ecology across the modern world to establish proxies that can be applied to fossil leaf assemblages5,6. These proxies provide important opportunities to reconstruct paleoclimate and paleoecology and contribute to a greater understanding of the complex interplay between various systems of the planet throughout its history. This article details the methods necessary for the use of two proxies: 1) the leaf mass per area reconstruction method to elucidate paleoecology, and 2) digital leaf physiognomy to reconstruct paleoclimate.
Leaf dry mass per area (MA) is a frequently measured plant trait in both neo- and paleobotany. The primary value of MA, especially for fossil reconstructions, is that it is part of the leaf economics spectrum, a coordinated axis of well-correlated leaf traits that includes leaf photosynthetic rate, leaf longevity, and leaf nutrient content by mass7. The ability to reconstruct MA from fossils provides a window into these otherwise inaccessible metabolic and chemical processes and ultimately can reveal useful information about plant ecological strategy and ecosystem function.
Royer et al.5 developed a method to estimate the MA of woody non-monocotyledonous (dicot) angiosperm fossil leaves based on the area of the leaf blade and the width of the petiole. Theoretically, the leaf petiole acts as a cantilever, holding the weight of the leaf in the optimal position3,4. The cross-sectional area of the petiole, which makes up the most significant component of beam strength, should, therefore, be strongly correlated with the mass of the leaf. By simplifying the shape of the petiole into a cylindrical tube, the cross-sectional area of the petiole can be represented with the petiole width squared, allowing leaf mass to be estimated from a two-dimensional fossil (for more detail, see Royer et al.5). Leaf area can be measured directly. Together, petiole width squared divided by leaf area (i.e., the petiole metric; Table 1) provides a good proxy for fossil MA and allows paleobotanists to step into modern trait-based ecology. MA reconstruction methods have also been expanded to broadleaf and petiolate gymnosperms5,8, herbaceous angiosperms8, and ferns9, which produced relationships that differ from the relationships observed for woody dicot angiosperms and from each other. An expanded woody dicot dataset and new regressions equations for reconstructing the variance and mean of MA at the site level allow the inference of the diversity of leaf economic strategies and what strategies are most prevalent, among woody dicot angiosperms in fossil floras10.
The relationship between physiognomic leaf traits and their climate has been noted for over a century11,12. Specifically, the physiognomy of woody dicot angiosperm leaves is strongly correlated with temperature and moisture13. This relationship has formed the basis for numerous univariate14,15,16,17 and multivariate6,18,19,20,21,22 leaf physiognomic proxies for terrestrial paleoclimate. Both univariate and multivariate leaf physiognomic paleoclimate methods have been widely applied to angiosperm-dominated fossil floras across all continents spanning the last ~120 million years of Earth's history (Cretaceous to modern)23.
Two fundamental observations utilized in leaf physiognomic paleoclimate proxies are 1) the relationship between leaf size and mean annual precipitation (MAP) and 2) the relationship between leaf teeth (i.e., outward projections of the leaf margin) and mean annual temperature (MAT). Specifically, the average leaf size of all woody dicot angiosperm species at a locality is positively correlated with MAP, and the proportion of woody dicot angiosperm species at a locality with toothed leaves, in addition to the size and number of teeth negatively correlate with MAT6,12,13,14,15,16,24.
A functional link between these leaf physiognomy-climate relationships is strongly supported by both theory and observation1,2,25. For example, although larger leaves provide greater photosynthetic surface area, they require greater support, lose more water through transpiration, and retain more sensible heat due to a thicker boundary layer 1,26,27. Thus, larger leaves are more common in wetter, hotter environments because water loss through increased transpiration effectively cools leaves and is less problematic. In contrast, smaller leaves in drier hot climates reduce water loss and avoid overheating instead by increasing sensible heat loss28,29. Details of what factors, or combination of factors, contribute most strongly to explaining functional links remain enigmatic for other leaf traits. For example, there have been several proposed hypotheses to explain the leaf teeth-MAT relationship, including leaf cooling, efficient bud packing, enhanced support and supply of thin leaves, guttation through hydathodes, and enhanced early season productivity30,31,32,33.
Most leaf physiognomic paleoclimate proxies rely on categorical division of leaf traits rather than quantitative measurements of continuous variables, leading to several potential shortcomings. The categorical approach excludes the incorporation of more detailed information captured by continuous measurements that are strongly correlated with climate (e.g., number of teeth, leaf linearity), which can reduce the accuracy of paleoclimate estimates6,20,34. Additionally, in some of the leaf trait scoring methods, the traits being categorically scored can be ambiguous, leading to issues in reproducibility, and some traits have limited empirical evidence to support their functional link to climate6,15,16,35,36.
To address these shortcomings, Huff et al.20 proposed digitally measuring continuous leaf traits in a method known as digital leaf physiognomy (DiLP). A key advantage of DiLP over previous methods is its reliance on traits that 1) can be measured reliably across users, 2) are continuous in nature, 3) are functionally linked to climate, and 4) display phenotypic plasticity between growing seasons6,37. This has led to more accurate estimates of MAT and MAP than previous leaf physiognomic paleoclimate methods6. In addition, the method accommodates the imperfect nature of the fossil record by providing steps to account for damaged and incomplete leaves. The DiLP method has been successfully applied to a range of fossil floras from multiple continents spanning a large range of geologic time6,38,39,40,41,42.
The following protocol is an expansion of that described in earlier work5,6,20,34. It will explain the procedures necessary to reconstruct paleoclimate and paleoecology from woody dicot angiosperms fossil leaves using the DiLP and MA reconstruction methods (see Table 1 for an explanation of the variables measured and calculated through the use of this protocol). In addition, this protocol provides steps to record and calculate leaf traits not included in DiLP or MA analysis but that are easy to implement and provide useful characterizations of leaf physiognomy (Table 1). The protocol follows the following format: 1) Imaging fossil leaves; 2) leaf digital preparation, organized into five possible preparation scenarios; 3) leaf digital measurement, organized into the same five possible preparation scenarios; and 4) DiLP and MA analyses, using the R package dilp10.
The protocol for MA reconstructions is embedded within the DiLP protocol because both are convenient to prepare for and measure alongside each other. If a user is interested in MA analyses only, they should follow the preparation steps described in DiLP preparation scenario 2, whether or not the leaf margin is toothed, and the measuring steps describing petiole width, petiole area, and leaf area measurements only. A user can then run the appropriate functions in the dilp R package that performs the MA reconstructions.
Protocol
1. Fossil leaf imaging
- Position the leaf fossil under the camera and ensure it is lying as flat as possible using, for example, a sandbox or putty to wedge under the fossil.
NOTE: When photographing multiple specimens on a single block, it is best to photograph them as close-ups separately to ensure details of the fossil are clear and sharp. It is also useful to place the fossil on a solid dark matte background, such as black felt or velvet. - Place a scale bar horizontally and in the same vertical plane as the leaf, placing it close to the fossil but not covering any parts of it. If there is little or no matrix surrounding the fossil, the scale should be placed within the photo frame and be in focus.
- Using a camera tripod or copy stand, position the camera directly above the fossil leaf with the lens parallel to the rock surface. To ensure the detail of the leaf is captured sharply, position the camera as close to the fossil as possible while staying within the focal distance of the lens/camera and ensuring the entire fossil is within the frame of the photograph.
NOTE: If possible, it is best to use a high-resolution digital camera and a macro lens with manual focus and enough depth of field to crisply focus on the leaf that will be processed. - Using indirect light, light the fossil as needed to clearly see the entire outline of the specimen. It is often necessary to readjust the lighting for each fossil.
- Photograph the fossil leaf and label the image file appropriately.
2. Digital preparation
NOTE: An illustration of leaf architectural terminology used throughout these protocols is provided in Figure 1. Use the decision tree (Figure 2) and provided examples (Figure 3) to determine which preparation scenario is applicable to the fossil leaf to be measured and proceed to that appropriate section. Reference Table 2 for additional considerations in the preparation steps. If the leaf falls under scenario 1 or 5, the leaf cannot be prepared for quantitative leaf physiognomy measurements.
- Scenario 2: Entire margined leaf whose area, or half area, is preserved or can be reconstructed.
- Open the file in the image processing software (e.g., Adobe Photoshop or GIMP). Crop the image, if necessary, which helps to reduce the final file size but ensures that the scale bar is still included.
- Double the width of the working area by clicking Image > Canvas (Photoshop); Image > Canvas Size (GIMP). Adding a new canvas to the right or left of the current canvas is suggested.
- If the leaf margin requires some reconstruction, decide whether leaf area and shape can be more reliably measured from a half leaf or whole leaf (Figure 3).
- Copy the leaf out of the rock matrix. Trace the whole or half leaf, including the petiole if present, using a lasso tool (see Table 2). Copy and paste the selection and place it in an open area of the canvas. Consider pasting two copies of this selection, one being an unedited one to return to if needed to restart the preparation process.
- Repair any damaged portions of the margin using a line of appropriate color (typically black if on a white background). Draw a line that spans the damaged margin so that the margin is reliably reconstructed, using, for example, the paintbrush or line tool. Be sure the line is thick enough to be seen (~1-2 pt weight) and that it connects the margin across the damaged area.
- Remove the petiole from the leaf, if present, using the lasso tool.
- Visually, follow the leaf margin along the base until the point it contacts the petiole, which is often darker in color and contains no distinctive veins. Place a lasso point there. Do the same on the other half of the leaf and place the second point there.
NOTE: If the leaf base is symmetrical, the line will be ~perpendicular to the petiole; if it's asymmetric, the line will be at an angle. - Encircle the entire petiole to finish the selection. Cut and paste, or use the move tool, to place the petiole next to the leaf blade, but not touching it.
NOTE: For a cordate or lobate leaf base, meaning the base extends below where the petiole attaches to the leaf blade, the petiole has the potential to rest upon the leaf base below where the petiole attaches to the leaf blade. Take care to cut the petiole out where it actually attaches, trace the petiole margin closely, and repair the resulting damaged margin. It is recognized that this may be difficult to see in most fossils.
- Visually, follow the leaf margin along the base until the point it contacts the petiole, which is often darker in color and contains no distinctive veins. Place a lasso point there. Do the same on the other half of the leaf and place the second point there.
- Crop the final area of the image if necessary to reduce file size. See Figure 3 for an example of how the completed prepared image should appear.
- Scenario 3: a toothed leaf whose area, or half area, cannot be reconstructed but has ≥ two consecutive teeth and ≥25% of the leaf preserved
NOTE: Tooth measurements are the only traits that can be measured on leaves of this category, so leaves are prepared only for these measurements.- Open the file in an image processing software (e.g., Adobe Photoshop or GIMP). Crop the image, if necessary, which helps to reduce the final file size but ensure the scale bar is still included.
- Tripe to quadruple the width of the working area by clicking Image > Canvas (Photoshop); Image > Canvas Size (GIMP). Adding a new canvas to the right or left of the current canvas is suggested.
- Copy the leaf out of the rock matrix. Trace the extent of the preserved leaf, including the petiole if present, using a lasso tool. Do not worry about tracing damaged portions of the margin precisely because they will be removed. Copy and paste the selection and place it in an open area of the canvas. Consider pasting two copies of this selection, one being an unedited version to return to if needed to restart the preparation process.
- If present, remove the petiole from the leaf using the using lasso tool.
- Visually, follow the leaf margin along the base until the point it contacts the petiole, which is often darker in color and contains no distinctive veins. Place a lasso point there. Do the same on the other half of the leaf and place the second point there.
NOTE: If the leaf base is symmetrical, the line will be ~perpendicular to the petiole, if it's asymmetric, the line will be at an angle. - Encircle the entire petiole to finish the selection. Cut and paste, or use the move tool, to place the petiole next to the leaf blade, but not touching it.
NOTE: For a cordate or lobate leaf base, meaning the base extends below where the petiole attaches to the leaf blade, the petiole has the potential to rest upon the leaf base below where the petiole attaches to the leaf blade. Take care to cut the petiole out where it actually attaches, trace the petiole margin closely, and repair the resulting damaged margin. This may be difficult to see in most fossils.
- Visually, follow the leaf margin along the base until the point it contacts the petiole, which is often darker in color and contains no distinctive veins. Place a lasso point there. Do the same on the other half of the leaf and place the second point there.
- Remove the area adjacent to damaged portions of the margin using a lasso tool.
- Begin the selection at a point along the margin that bounds the damaged portion and draw a straight line from that point to the major vein that is perpendicular to that major vein (Figure 4). Start the selection at the preserved primary tooth sinus closest to the damage. This ensures that the flank of a tooth is not included as an internal perimeter in subsequent measurements, and subsidiary teeth are not measured as if they are primary teeth. This may not be appropriate if teeth are distantly spaced, as too much preserved margin may end up being removed (Figure 4).
- Proceed the selection along the major vein until at level with the other bound of the damaged margin, and draw a straight line perpendicular to the major vein to the margin (Figure 4).
NOTE: For pinnate leaves (Figure 1A), with and without agrophic veins (see Ellis et al.43), the major vein is the primary vein (i.e., midvein). For palmately veined leaves (Figure 1B,D), the major vein is the nearest primary vein (e.g., Figure 4B). For pinnately lobed leaves (Figure 1C), if the damage is located on a pinnate lobe, the major vein is the vein (typically a secondary vein) that feeds the lobe. - Complete the selection and delete this portion of the leaf. Repeat for all damaged portions of the leaf.
- Copy and paste this prepared leaf and place it in an open area of the canvas.
- Remove the teeth using a lasso tool.
- Start at the leaf apex, one of the lobe apices, or the most apical tooth of a leaf fragment, and make a selection at each primary tooth sinus along that leaf, lobe, or fragment (Figure 5; see Supplementary Figure 1, Supplementary Figure 2 for tips on how to distinguish primary and subsidiary teeth and how to distinguish teeth from lobes). Be sure to follow the appropriate rules when cutting out teeth (Table 2; Supplementary Figure 3).
NOTE: Primary teeth often become smaller towards the base and apex. - After selecting the apical sinus of the most basal tooth, apply the extension rule (Table 2; Supplementary Figure 4) to cut the last tooth of the sequence out.
- Remove the teeth by cutting and pasting the teeth next to the leaf blade with the teeth removed without touching it. If the preparation has additional leaf lobes or fragments that require teeth to be removed, repeat the above steps until all teeth are removed.
- Start at the leaf apex, one of the lobe apices, or the most apical tooth of a leaf fragment, and make a selection at each primary tooth sinus along that leaf, lobe, or fragment (Figure 5; see Supplementary Figure 1, Supplementary Figure 2 for tips on how to distinguish primary and subsidiary teeth and how to distinguish teeth from lobes). Be sure to follow the appropriate rules when cutting out teeth (Table 2; Supplementary Figure 3).
- If an extra version of the original cut-out leaf was created, delete the extra version. Crop the final area of the image if necessary to reduce file size. See Figure 3 for an example of how the completed prepared image should appear.
- Scenario 4: a toothed leaf whose area, or leaf area, is preserved or can be reconstructed
- Open the file in the image processing software (e.g., Adobe Photoshop or GIMP). Crop the image, if necessary, which helps to reduce the final file size but ensure the scale bar is still included.
- Tripe to quadruple the width of the working area by clicking Image > Canvas (Photoshop); Image > Canvas Size (GIMP). Adding a new canvas to the right or left of the current canvas is suggested.
- Decide how the leaf will be prepared. Leaf area/shape measurements need to be made on a whole leaf or half leaf, decide which option will result in more accurate measurements. Tooth measurements should be made along all sections of preserved margin. In some cases, the leaf area/shape measurements may occur on a different subset of the leaf than the subset where tooth variables are measured.
NOTE: In the provided example (Figure 6), it was decided that a half leaf would be more reliably reconstructed than a whole leaf. The preserved margin on the bottom right (>1 preserved tooth) was included for the tooth measurements. The following protocol for Scenario 4 roughly follows the provided example (Figure 6), but details may vary slightly in different preparation contexts. - Copy the leaf out of the rock matrix, making sure to include all preserved margin.
- Trace the margin of the leaf, including the petiole if present, using a lasso tool. Do not trace damaged portions of the margin that will not be included in area/shape measurements precisely because they will be removed (e.g., right half of the leaf in Figure 6).
- Copy and paste the selection, and place in an open area of the canvas. Consider pasting two copies of this selection, one being an unedited to return to if needed to restart the preparation process.
- If present, remove the petiole from the leaf using the lasso tool.
- Visually, follow the leaf margin along the base until the point it contacts the petiole, which is often darker in color and contains no distinctive veins. Place a lasso point there. Do the same on the other half of the leaf and place the second point there.
NOTE: If the leaf base is symmetrical, the line will be ~perpendicular to the petiole; if it's asymmetric, the line will be at an angle. Encircle the entire petiole to finish the selection. - Cut and paste, or use the move tool, to place the petiole next to the leaf, but not touching it.
NOTE: For a cordate or lobate leaf base, meaning the base extends below where the petiole attaches to the leaf blade, the petiole has the potential to rest upon the leaf base below where the petiole attaches to the leaf blade. Take care to cut the petiole out where it attaches, trace the petiole margin closely, and repair the resulting damaged margin (Supplementary Figure 5). This may be difficult to see in most fossils.
- Visually, follow the leaf margin along the base until the point it contacts the petiole, which is often darker in color and contains no distinctive veins. Place a lasso point there. Do the same on the other half of the leaf and place the second point there.
- Copy and paste the isolated leaf with the petiole removed to create a second copy to prepare for tooth measurements and place it in an open area of the canvas.
- Prepare a version of the leaf for leaf area and shape measurements.
- If preparing a half leaf, trim excess leaf material off using the lasso tool so only a complete half leaf remains. If preparing a complete leaf, do not remove any leaf material.
- If necessary, repair any damaged areas along the margin using a line of appropriate color using a line or paintbrush tool (typically a black line for a white background). Be sure the line is thick enough to be seen (~1-2 pt weight) and that it connects the margin across the damaged area.
- Prepare a version of the leaf for tooth measurements.
- Remove the area adjacent to damaged portions of the margin using a lasso tool.
- Begin selection at a point along the margin that bounds the damaged portion and draw a straight line from that point to the major vein that is perpendicular to that major vein (Figure 4). Start the selection at the preserved primary tooth sinus closest to the damage. This ensures that the flank of a tooth is not included as an internal perimeter in subsequent measurements, and subsidiary teeth are not measured as if they are primary teeth. This may not be appropriate if teeth are distantly spaced, as too much preserved margin may end up being removed (Figure 4).
- Proceed the selection along the major vein until at level with the other bound of the damaged margin and draw a straight line perpendicular to the major vein to the margin (Figure 4).
NOTE: For pinnate leaves (Figure 1A), with and without agrophic veins (see Ellis et al.43 for definition and examples), the major vein is the primary vein (i.e., midvein). For palmately veined leaves (Figure 1B,D), the major vein is the nearest primary vein (e.g., Figure 4B). For pinnately lobed leaves (Figure 1C), if the damage is located on a pinnate lobe, the major vein is the vein (typically a secondary vein) that feeds the lobe. - Delete the damaged portion of the leaf. Do the same for all damaged portions of the leaf.
- Remove the area adjacent to damaged portions of the margin using a lasso tool.
- Copy and paste the version prepared for tooth measurements, with damaged portions removed, and place in an open area of the canvas.
- Remove the teeth using a lasso tool.
- Start at the leaf apex, one of the lobe apices, or the most apical tooth of a leaf fragment, and make a selection at each primary tooth sinus along the leaf, lobe, or fragment (Figure 5; see Supplementary Figure 2 for tips on how to distinguish primary and subsidiary teeth and how to distinguish teeth from lobes). Be sure to follow the appropriate rules when cutting out teeth (Table 2; Supplementary Figure 2).
NOTE: Primary teeth often become smaller towards the base and apex. - After selecting the apical sinus of the most basal tooth, apply the extension rule (Table 2; Supplementary Figure 4) to cut the last tooth of the sequence out.
- Remove the teeth by cutting and pasting the teeth next to the leaf blade with the teeth removed without touching it. If the preparation has additional leaf lobes or fragments that require teeth to be removed, repeat the above steps until all teeth are removed.
- Start at the leaf apex, one of the lobe apices, or the most apical tooth of a leaf fragment, and make a selection at each primary tooth sinus along the leaf, lobe, or fragment (Figure 5; see Supplementary Figure 2 for tips on how to distinguish primary and subsidiary teeth and how to distinguish teeth from lobes). Be sure to follow the appropriate rules when cutting out teeth (Table 2; Supplementary Figure 2).
- If an extra version of the original cut out leaf was created, delete the extra version. Crop the final area of the image if necessary to reduce file size. See Figure 3 for an example of how the completed prepared image should appear.
3. Digital measurement
NOTE: A data entry template spreadsheet is provided as Supplementary File 1. Reference Table 3 for additional considerations in the measurement steps. In scenarios 1 and 5, the only step required is to record the leaf margin state in the data entry spreadsheet (step 3.5).
- Open ImageJ software44. Set which measurements will automatically be made (do this once after the program is installed).
- Click Analyze > Set Measurements, and select only Area, Perimeter, and Feret's diameter. Make sure decimal places are set to 3.
- Open the prepared fossil leaf image by clicking File > Open or simply by dragging and dropping the image into the already opened ImageJ toolbar.
- Set the scale for every new leaf image.
NOTE: This is a critical step and must be done for every new leaf image to ensure accurate measurements.- Click on the Straight line tool. Zoom in on the scale bar and draw the longest straight line possible across the scale bar.
- Click Analyze > Set Scale. In known distance, enter the length measured in cm (to be consistent with the unit used in the modern calibration dataset). It is not necessary to change the unit of length. Click OK.
- Mark the leaf as toothed (0) or entire (1) in the data entry spreadsheet.
- Measure petiole width if the petiole is present. Measurements should be made on the original copy of the leaf still in the rock matrix, as it provides much better context.
NOTE: If the petiole is not present, in some cases, the width of the midvein at its basalmost position can be measured instead of the petiole. However, this should only be done if the entire width of the midvein is preserved (i.e., there is no lamina compressed atop the vein or the fossil preserves the abaxial side of the leaf) and other specimens from the same species or morphotype shows that basal vein width is equivalent to petiole width.- Draw a straight line perpendicular to the petiole, where the petiole meets the leaf blade, or if the point of insertion is asymmetric, draw a line perpendicular to the petiole at the most basal point of insertion. It is important to draw this line carefully. Thus, it is recommended to zoom in on this area of the leaf to make it easier to draw the line precisely.
NOTE: There are special circumstances where this step needs to be modified, including if, at the most basal point of insertion, there exists damage, trichomes, nectaries, thorns, or other features that prevent accurate measurements of petiole width. In these cases, measure the petiole width at the first point below the feature where the measurement can be reliably made. - Click Analyze > Measure, or use a shortcut to measure the length of the line drawn. Draw the same line on the image to create a record of exactly where the measurement was made by clicking Edit > Draw or using a shortcut. Change the color of the line using a tool on the main toolbar (color picker), if necessary.
- Once the line is drawn, save the image, preferably under a modified file name.
- Record the length of this line under petiole width in the data entry spreadsheet.
- Draw a straight line perpendicular to the petiole, where the petiole meets the leaf blade, or if the point of insertion is asymmetric, draw a line perpendicular to the petiole at the most basal point of insertion. It is important to draw this line carefully. Thus, it is recommended to zoom in on this area of the leaf to make it easier to draw the line precisely.
- Prepare leaf for additional measurements by making the image black and white. To do so, click Image > Type > 8 bit.
- Threshold the image by clicking Image > Adjust > Threshold or use the shortcut. A box titled Threshold will open and change part of the image to red. If the leaf is light in color and the background is dark, click Dark Background.
- Adjust the threshold using the slider bar until the interior of the leaf is red and is distinct from the background. This is a critical step and an easy place to produce imprecise data. Make sure that the red area corresponds exactly to the leaf (i.e., all the perimeter of the leaf is red and no more), by zooming in on some sections of the margin. Gaps of red within the leaf interior are acceptable and do not affect the measurements.
NOTE: If the outline of the leaf is not well defined, first attempt to adjust the threshold while zoomed in to confirm that the outline of the leaf is red. If poor contrast between the fossil and the background prevents a reliable threshold from being applied, use the paintbrush tool to add a solid outline to the leaf perimeter in areas where contrast is too poor. Alternatively, return the leaf to the image processing software (e.g., Adobe Photoshop or GIMP) and adjust the contrast of the isolated leaf layers or the color of the background to better differentiate them. - Measure leaf area and shape for leaves prepped in scenarios 2 (step 2.1) and 4 (step 2.4).
NOTE: Use Figure 2 and Figure 6B as a guide for which variables are measured on which components of the prepared image. If leaves were prepared in scenario 3 (step 2.2), skip this step and proceed to step 3.11.- Measure the leaf prepared for leaf area and shape measurements, which should have only its petiole removed (if a petiole was present). Select the Wand tool. Click on the interior of the leaf. The entire leaf should be outlined in yellow—confirm the outline is correct.
- Make measurements by clicking Analyze > Measure or using the shortcut.
- If the area measured is prepared as a whole leaf, record area, perimeter, Feret, and minimum Feret in the data entry spreadsheet. If area measured is prepared as half leaf, only record Feret and proceed to next step.
- If the area measured is a half leaf, measure the artificial middle perimeter of the leaf, which is the length of the artificial perimeter that results from cutting the leaf in half (Figure 6B). If the area measured is the whole leaf, skip this step and step 3.10.5.
NOTE: Measuring the artificial middle perimeter allows the blade perimeter to be calculated from half leaves (see step 3.10.5 below). Blade perimeter is not used in variables included in DiLP and MA analyses but is used for other variables useful for physiognomy characterization (e.g., shape factor, compactness; Table 1).- Select the segmented line tool by right clicking the Line tool. Trace the entire length of the artificial middle perimeter.
- Click Analyze > Measure or use the shortcut to measure the length. This measurement will be used in the formula to calculate blade perimeter below (step 3.10.5).
- If the area measured is a half leaf, modify measurements as you enter them in the data entry spreadsheet by multiplying the area by 2, multiplying the minimum Feret by 2, and calculating the blade perimeter by first subtracting the artificial middle perimeter from the half leaf perimeter and then multiplying by 2 using the following formula:
Blade perimeter = (perimeter – artificial middle perimeter) x 2 - If a cut-out petiole is present, measure its area. If not present, measuring is completed for scenario 2, but proceed to step 3.11 for scenario 4.
- Click the cut-out petiole with the wand tool. The petiole should be outlined in yellow. Make measurements by clicking Analyze > Measure or using the shortcut. Record area under petiole area in the data entry spreadsheet.
- For scenario 2 (step 2.1), measuring is now completed; for scenario 4 (step 2.3), proceed to the next step.
- Measure tooth variables for leaves prepped in scenarios 3 (step 2.2) and 4 (step 2.3).
- Measure the raw blade. With the wand tool, select the interior of the raw blade (i.e., leaf prepared for tooth measurements that still has its teeth; Figure 6B). It should be outlined in yellow. Make measurements by clicking Analyze > Measure or using the shortcut.
NOTE: Depending on how the leaf was prepped, it may be necessary to measure several disjunct sections, adding their areas together (e.g., Figure 6B). Alternatively, you can select multiple sections at a time by selecting a second section with the wand tool while holding the Shift key. - Record area and perimeter under raw blade area and raw blade perimeter in the data entry spreadsheet.
- Measure the internal raw blade. Select the interior of the internal raw blade (i.e., leaf prepared for tooth measurements that has the teeth removed; Figure 6B). It should be outlined in yellow. Make measurements by clicking Analyze > Measure or using the shortcut.
NOTE: Depending on how the leaf was prepped, it may be necessary to measure several disjunct sections, adding their areas together (e.g., Figure 6B). Alternatively, you can select multiple sections at a time by selecting a second section with the wand tool while holding the Shift key. - Record area and perimeter under internal raw blade area and internal raw blade perimeter in the data entry spreadsheet.
- Measure the length of cut perimeter. Remove the threshold to see the leaf clearly, click Reset in the threshold box or click Edit > Undo—the latter will usually also remove the black and white 8-bit conversion. Select the segmented line tool and trace the full length of the cut perimeter on the raw blade.
- Measure by clicking Analyze > Measure or using the shortcut. If there are multiple portions, repeat the previous steps to measure the length of cut perimeter of each portion. Record the length, or the sum of lengths, under length of cut perimeter in the data entry spreadsheet.
NOTE: The cut perimeter is introduced through preparation of the leaf by removing damage. In most cases, this is different from the artificial middle perimeter (Figure 6B). - Count primary and subsidiary teeth, if present.
NOTE: See Supplementary Figure 2 for tips on how to distinguish between primary and subsidiary teeth.- If the threshold is not already removed, remove it now. To remove the threshold, click Reset in the threshold box or click Edit > Undo—the latter will usually also remove the black and white 8-bit conversion.
- Count the number of primary teeth (see Supplementary Figure 2 for tips on how to distinguish primary and subsidiary teeth). Select the Multi-Point Tool. It may be necessary to right-click the Point Tool first, to select the Multi-Point Tool. Click on each primary tooth to number it.
- To remove a point selected by mistake, press Alt key (Windows OS) or Command/cmd or option (Mac OS) while at the same time clicking the point. Record the final number under # of primary teeth in the data entry spreadsheet.
- Clear the multi-point tool counts and annotations by clicking Edit > Selection > Select None or use the keyboard shortcut.
- Count the total number of teeth (i.e., all primary and subsidiary teeth present on leaf). Select the Multi-Point Tool. It may be necessary to right click the Point Tool first, to select the Multi-Point Tool. Click on each tooth, including primary and subsidiary, to number it.
NOTE: Counting the total number of teeth, rather than the number of subsidiary teeth, ensures that no teeth are double counted. The total number of teeth is subtracted by the number of primary teeth to determine the number of subsidiary teeth (see step 3.11.7.6). - To remove a point selected by mistake, press Alt key (Windows OS) or Command/cmd or option (Mac OS) while at the same time clicking the point. Subtract the number of primary teeth from the total number of teeth to determine the number of subsidiary teeth. Record this under # of subsidiary teeth in the data entry spreadsheet.
NOTE: Some users prefer to make tooth counts when preparing leaf images rather than when measuring.
- Measure the raw blade. With the wand tool, select the interior of the raw blade (i.e., leaf prepared for tooth measurements that still has its teeth; Figure 6B). It should be outlined in yellow. Make measurements by clicking Analyze > Measure or using the shortcut.
4. Running analyses in R software
NOTE: The following steps require the R package dilp11. The data entry spreadsheet is read into R and used by the package. Refer to the Additional Instructions tab in the data entry spreadsheet (Supplementary File 2). The R script can accommodate the analysis of multiple sites simultaneously or a single site.
- Open R using your preferred environment (R Studio is recommended). For an introduction to R, see, for example, https://cran.r-project.org/doc/manuals/r-release/R-intro.pdf.
- Install the dilp package in your R session. See the following website for more information on how to install the package and run its associated functions: https://cran.r-project.org/package=dilp
- Read in the .csv file containing the fossil woody dicot angiosperm leaf trait data (i.e., data recorded in the data entry spreadsheet).
- Run the function dilp() for mean annual temperature (MAT) and mean annual precipitation (MAP) reconstructions with associated error. Results for MAT and MAP are reported from a multiple linear regression model (MLR; i.e., DiLP) and two single linear regression models (SLR; i.e., leaf area and margin analyses). Run the function lma() for leaf mass per area (MA) reconstructions at the morphotype and site levels.
- After running dilp(), it is recommended to check for potential data collection issues and confirm data quality by looking at the outliers and error objects in the returned dilp() results. Alternatively, use the function dilp_processing() followed by dilp_outliers() and dilp_errors(). Address any flagged issues by referencing the prepared specimen and potentially remeasuring it. It is recommended that the original data file be edited and then re-read back into R.
- Determine if the fossil site falls within the leaf physiognomic multivariate space of the calibration data set using the function dilp_cca().
Representative Results
A previously published dataset of leaf physiognomy measurements from the early Eocene McAbee fossil site in south-central British Columbia was used to provide an example of representative results using both the digital leaf physiognomy (DiLP) and leaf mass per area (MA) reconstruction methods (Lowe et al.38; data provided in Supplementary File 2). The site provides an opportunity to reconstruct paleoclimate and paleoecology during the warmest interval of the Cenozoic (the Early Eocene Climatic Optimum) in an upland and volcanic landscape38,45,46,47. Fossil assemblages were sampled from two separate horizons in a lacustrine sequence, named H1 (28 cm thick) and H2 (27 cm thick), pooled over a narrow range of stratigraphy using a census technique, whereby all specimens able to be assigned a morphotype were collected or counted38,48.
The McAbee leaf physiognomic data passed the error checks flagged by dilp_errors(), and seven outliers flagged by dilp_outliers() were double-checked to ensure the values represent true variation in data and not a methodological mistake. The data was subsequently run through the dilp() function to produce paleoclimate and the lma() function for leaf mass per area reconstructions.
MA reconstructions and the lower and upper bounds of their 95% prediction intervals are reported in Table 4 at both the species- and site level, using equations presented in Royer et al.5 and Butrim et al.10. Reconstructed values are within the range of MA typical for modern terrestrial species (30-330 g/m2)49. Using thresholds discussed in Royer et al.5, most species have a reconstructed MA that aligns with leaf life spans of <1 year (≤87 g/m2), some ~1 year (88-128 g/m2), while none are typical of >1 year (≥129 g/m2). Reconstructions of site MA mean, and variance at McAbee reflect the prevalence and diversity of leaf economic strategies at a site10,50. There are no prominent differences between site mean and variance between H1 and H2, and thus, there is no evidence that the composition and diversity of leaf economic strategies varied between the two points in time. Additionally, the site-mean reconstructions made using the equations of Royer et al.5 and Butrim et al.10 were very similar.
Reconstructions of mean annual temperature (MAT) and mean annual precipitation (MAP) using multiple linear regression (DiLP) and single linear regression (leaf margin and leaf area analyses) equations presented in Peppe et al.6 are shown in Table 5. Paleoclimate estimates are most reliably inferred if the leaf physiognomy of the fossil leaf assemblages occurs within the physiognomic space of the calibration dataset. This is assessed through the canonical correspondence analysis (CCA) analysis step carried out by the function dilp_cca(). Both McAbee H1 and H2 fall within the range of leaf physiognomy observed in the calibration dataset (Figure 7A). If sites had reconstructed values that fell outside calibration space, paleoclimate reconstructions should be interpreted cautiously (e.g., through comparison to independent lines of evidence; see Peppe et al.6 for further discussion). Reconstructed MAT and MAP for both H1 and H2 are consistent with a temperate seasonal biome (Figure 7B,C), which agrees well with independent lines of evidence, including nearest living relative based inferences of both the fossil floral and insect communities at McAbee45.
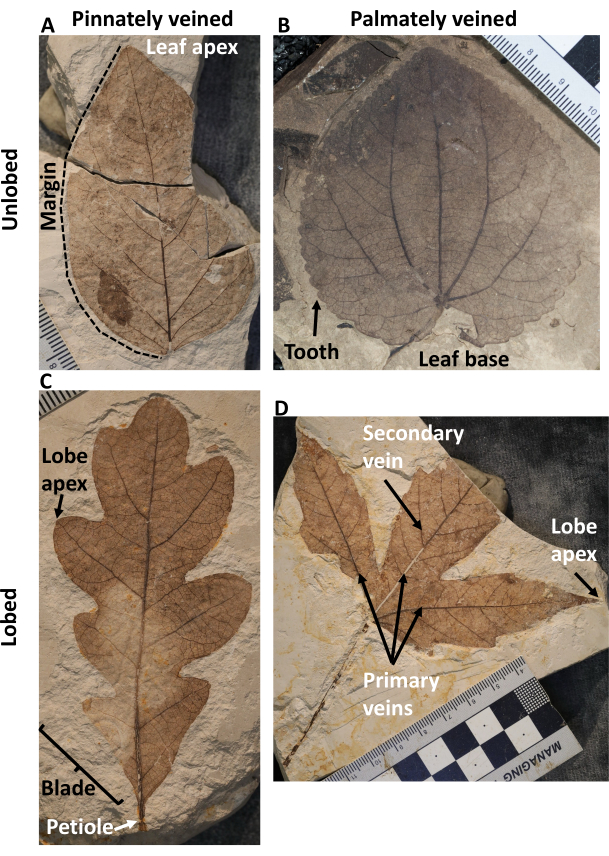
Figure 1: Leaf physiognomy and architectural terminology throughout this article. (A) A pinnately veined, unlobed, and entire-margined leaf, (B) a palmately veined, unlobed, and toothed leaf, (C) a pinnately veined, lobed, and entire-margined leaf, (D) a palmately veined, lobed, and toothed leaf. Please click here to view a larger version of this figure.
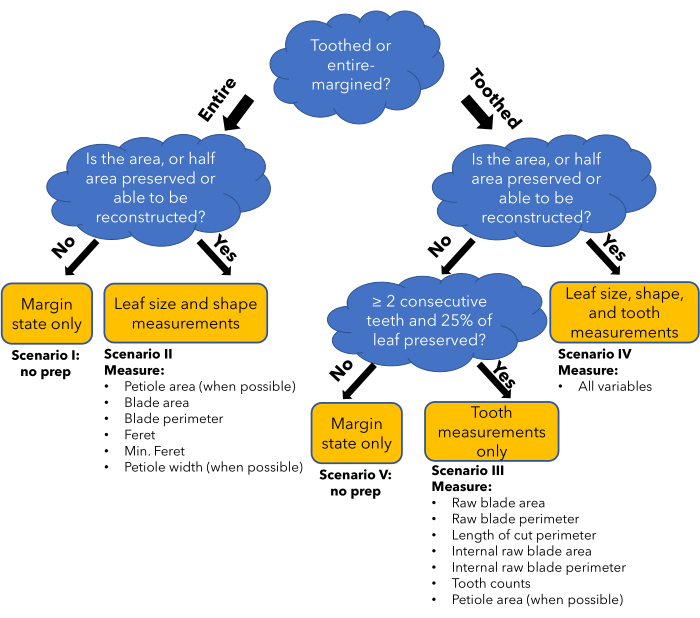
Figure 2: Flow chart of the method. A flowchart demonstrating how different leaf preservation conditions and leaf types determine what general type of leaf traits can be measured reliably (yellow box). This determines which preparation scenario will be followed in the protocol, and in which columns data will be entered in the data entry spreadsheet (bullet points). Please click here to view a larger version of this figure.
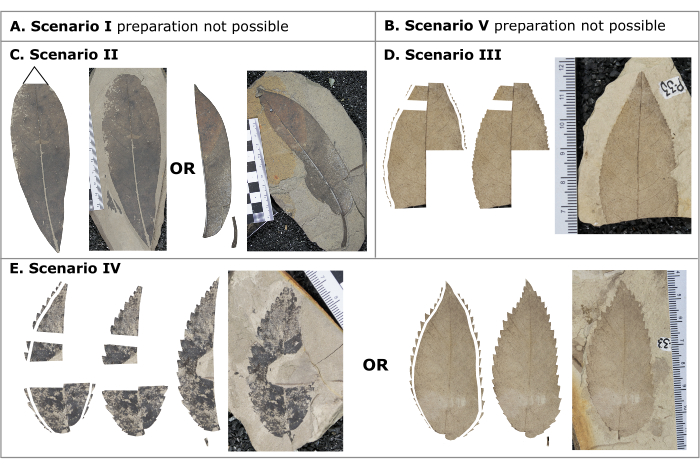
Figure 3: Different preparation scenarios. Different preparation scenarios demonstrating examples of completed digitally prepared images ready for the measurement phase. (A) Scenario 1, entire margined leaf whose area, or half area, cannot be reconstructed, (B) Scenario 5, toothed leaf whose area, or half area, cannot be reconstructed and does not have ≥2 consecutive teeth and/or ≥25% of the leaf preserved, (C) Scenario 2, entire margined leaf whose area, or half area, is preserved or can be reconstructed, (D) Scenario 3, a toothed leaf whose area, or half area, cannot be reconstructed but has ≥2 consecutive teeth and ≥25% of the leaf preserved, (E) Scenario 4, a toothed leaf whose area, or half area, is preserved or can be reconstructed. Please click here to view a larger version of this figure.

Figure 4: Illustration of damage removal. Illustrating how to cut out damaged margin, and the leaf area adjacent to that damaged margin. Dashed red lines demonstrate how selections are made with the lasso tool. Note that the bounds of damage were intentionally started at the sinuses of primary teeth (see Supplementary Figure 2 for help differentiating primary from subsidiary teeth). (A) A pinnately veined leaf where the selection is extended to the mid-vein. (B) A palmately veined leaf where the selection is extended to the nearest primary vein. Please click here to view a larger version of this figure.
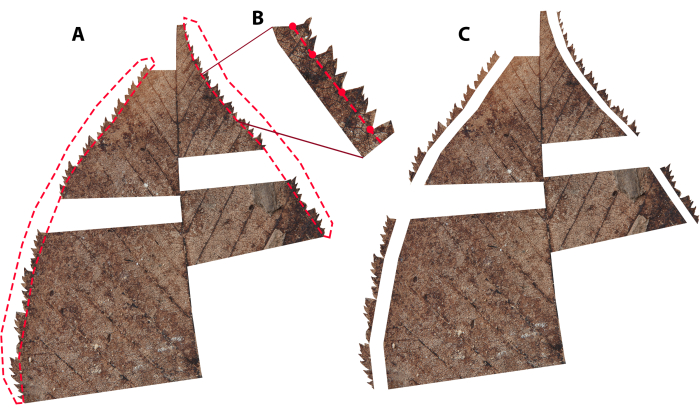
Figure 5: Illustrating an example of how to cut out teeth. (A) Dashed red lines demonstrate how selections are made with the lasso tool. Note that in this case, the teeth are compound, so selections were made between primary sinuses only (see Supplementary Figure 2 for help differentiating primary from subsidiary teeth), (B) a zoomed-in perspective of how teeth selection was made, with red dots representing where the mouse was clicked during selection, (C) the copy of the leaf when the teeth are removed. Please click here to view a larger version of this figure.
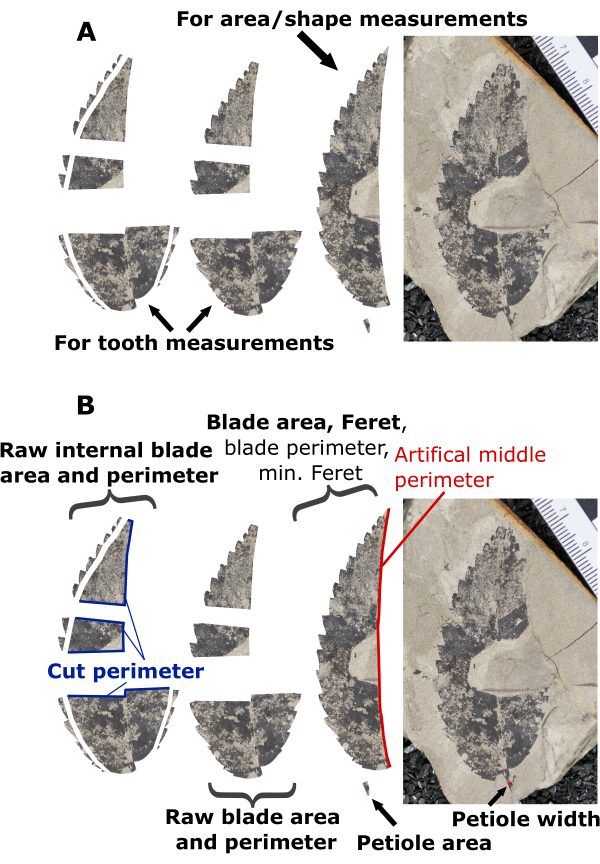
Figure 6: Illustration of preparation scenario 4. Illustration of preparation decisions and measuring steps for an example leaf prepared in scenario 4. (A) A preparation scenario where it was decided that a half leaf provided the most reliable leaf shape and area measurements, and preserved margins on both medial halves were included for tooth measurements. (B) An example demonstrating which variables are measured on various components of the prepared leaf. Bolded text highlights measurements needed for DiLP and MA analyses, while non-bolded text (blade perimeter, minimum Feret, and artificial middle perimeter) highlights measurements that are not required but useful for additional physiognomic characterizations (e.g., shape factor and compactness; Table 1). Please click here to view a larger version of this figure.
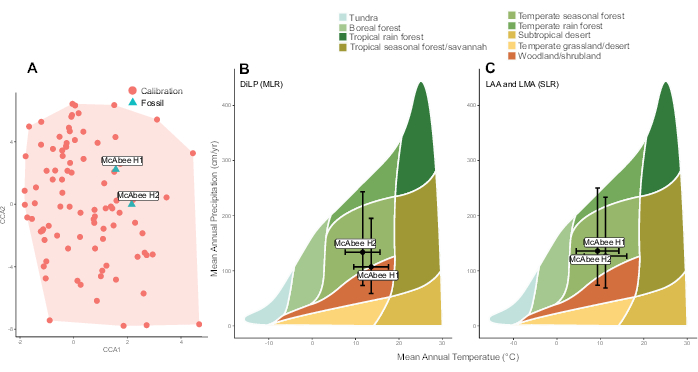
Figure 7: Representative results. Results from two fossils horizons (H1 and H2) sampled at the early Eocene McAbee Fossil Beds from Lowe et al.38. (A) Canonical correspondence analysis showing the representation of multivariate leaf physiognomy in the calibration dataset. Calibration data is from Peppe et al.6. The leaf physiognomy of the two McAbee horizons are overlain and occur within the calibration space. (B and C) Temperature and precipitation estimates, and their associated uncertainty (standard errors of the models), using equations presented in Peppe et al.6 of the two McAbee horizons overlain on a Whittaker Biome diagram. (B) Estimates reconstructed using the Digital Leaf Physiognomy (DiLP) multiple linear regressions models (MLR), (C) Estimates reconstructed using the leaf area analysis (LAA) and leaf margin analysis (LMA) single linear regressions (SLR) equations of the two McAbee horizons overlain on a Whittaker Biome diagram. Please click here to view a larger version of this figure.
Table 1: Leaf physiognomic variables. Variables that are measured and/or calculated and applied in predictive models using this protocol to reconstruct leaf dry mass per area (MA), mean annual temperature (MAT), and mean annual precipitation (MAP). MAT and MAP are reconstructed with equations presented in Peppe et al.6 using a multivariate approach for Digital Leaf Physiognomy (DiLP) and univariate approaches for leaf margin analysis (LMA) and leaf area analysis (LAA). Variables listed as Other are not used in MA, DiLP, LMA, and LAA analyses but are still measured and calculated using this protocol because they are easy to implement and provide useful characterizations of leaf physiognomy. Please click here to download this Table.
Table 2: Additional considerations and explanations for preparation steps. Please click here to download this Table.
Table 3: Additional considerations and explanations for measuring steps. Please click here to download this Table.
Table 4: Reconstructions of leaf dry mass per area (MA) and associated upper and lower bounds of the 95% prediction intervals for McAbee Fossil Beds from Lowe et al.38. Reconstructions are made for morphotype mean5, site mean5,10, and site variance10. Please click here to download this Table.
Table 5: Reconstructions of mean annual temperate (MAT) and mean annual precipitation (MAP) for Horizon 1 (H1) and 2 (H2) at the early Eocene McAbee Fossil Beds using the multiple linear regressions (MLR) of Digital Leaf Physiognomy (DiLP) and the single linear regressions (SLR) of leaf margin analysis (LMA) and leaf area analysis (LAA) presented in Peppe et al.6. Please click here to download this Table.
Supplementary Figure 1: Quercus rubra leaf from Harvard Forest illustrating the lobe vs. tooth rule. Line segments p and d are defined in text. Scale bars = 1 cm. Please click here to download this File.
Supplementary Figure 2: Betula lutea leaf from Harvard Forest illustrating the rules for differentiating subsidiary teeth from primary teeth. The isolated leaf segment has been magnified 2X. The blue line connects sinuses with the greatest degree of incision (i.e., primary sinuses), and teeth associated with these sinuses are considered primary (blue arrows). Red dots mark teeth that can be differentiated as a subsidiary because their apical sinuses are incised to a lesser degree. Teeth denoted by the red arrows have a similar degree of incision compared to the primary teeth but can be identified as subsidiary by a relatively thinner gauged principal vein compared to the primary teeth. Scale bars = 1 cm. Please click here to download this File.
Supplementary Figure 3: Illustration of tooth selection, the pinnate lobe rule, and the lobe priority rule. (A) Tooth selection for a Hamamelis virginiana leaf from Huyck Preserve. The darkened areas correspond to leaf tissue that is included in total tooth selection because subsidiary teeth are differentiated from primary teeth. (B) Quercus alba leaf from IES illustrates the lobe priority rule. The darkened areas are measured as lobes, and the undarkened are measured as teeth, but all projections are considered lobes via the lobe priority rule. Scale bars = 1 cm. Please click here to download this File.
Supplementary Figure 4: Acer saccharum leaf from Allegheny National Forest illustrating the extension and solitary tooth rules. Dashed lines depict tooth selections. The solid line depicts the axis of symmetry for the associated tooth. The black area is a weight used to flatten the leaves for photography. Scale bars = 1 cm. Please click here to download this File.
Supplementary Figure 5: Illustrating the ideal way to cut a petiole out that is positioned on top of a cordate base. Please click here to download this File.
Supplementary File 1: Data entry template for all measured digital leaf physiognomy variables. This file should not be modified, as it will be used as the input file for the R package. Please click here to download this File.
Supplementary File 2: Example data from McAbee fossil beds from Lowe et al.38. This data was used to generate Figure 7 and for the discussion of representative results. Please click here to download this File.
Supplementary File 3: Rules document for fossil digital leaf physiognomy. Please click here to download this File.
Discussion
This article presents how continuous traits of leaf physiognomy can be measured on woody dicot angiosperm fossil leaves and subsequently applied to proxies developed from modern calibration data to reconstruct paleoclimate and paleoecology. This requires that care be taken to align methodological steps with those represented in the proxy calibration datasets5,6,10. This consideration starts before the application of this protocol during fossil leaf collection, particularly with respect to sample size. Pooling fossil leaf assemblages across as narrow a range of stratigraphy as possible to obtain a suitable number of measurable specimens and morphotypes to minimize time averaging is recommended. Limiting paleoclimate reconstruction to sites with at least 350 identifiable specimens and at least 15-20 woody dicot angiosperm morphotypes is also recommended19,51,52. Further, when choosing leaves for analyses, measuring as many leaves per morphotype as possible and, at minimum, choosing specimens that represent the variability of leaf physiognomy within a morphotype is recommended.
Further care must be taken while implementing the preparation and measuring sections to remain consistent with the calibration dataset. Steps carried out during the preparation stages have the greatest potential for subjectivity and varied results between users. However, if the protocol is followed deliberately and the additional considerations tables (Table 2, Table 3) and rules document (Supplementary File 3) are referenced often, this method results in objective and reproducible measurements of leaf physiognomy. For users new to the method, confirming that the leaves have been prepared correctly with someone who has more experience is suggested. Special care needs to be taken when measuring petiole width for MA reconstructions. Because these values are squared, inaccuracy in the measurements will become exaggerated. Incomplete preservation and damage can alter petiole dimensions and should be carefully avoided.
There are some limitations to these methods that are worth noting. Most importantly, the proxy reconstructions included in the dilp R package are for woody dicot angiosperms only and, thus, may exclude other plant groups that were prominent components of ancient communities. However, additional leaf petiole-based proxies for species-level MA have been published for petiolate and broadleaf gymnosperms5,8, herbaceous angiosperms8, and ferns9, which a user could incorporate separately if desired. The exclusion of prominent plant groups in communities beyond woody dicot angiosperms is likely most impactful to reconstructions of site-level MA mean and variance, as they will provide an incomplete perspective of economic strategies within the entire community. Phylogenetic history influences the occurrence of leaf teeth23, introducing the potential that analyzing fossil communities with novel taxonomic composition may impart uncertainty in resulting estimates, though the realization of this potential influence has not yet been tested and demonstrated.
Fossil leaves also need to be adequately preserved to incorporate quantitative measurements of leaf physiognomy beyond the margin state. For DiLP, this is especially true for entire-margined leaves, as they can only contribute information beyond the margin state if the whole leaf, or half leaf, is preserved or can be reconstructed. Similarly, leaves can only be incorporated into MA reconstructions if (1) both their petiole at its insertion to the leaf blade is preserved or, in specific cases, if the base of the leaf and the basal most portion of the midvein is preserved (see Note in step 3.6), and (2) if the size of the leaf can be estimated, either through whole leaf measurement or half leaf reconstruction. This means some morphotypes may be excluded altogether from site-level MA analyses. Lastly, time is a limitation with this protocol, as univariate alternatives for paleoclimate reconstructions take comparatively less time to produce.
Despite these limitations, the use of DiLP and MA reconstruction methods still has several advantages over other methods. MA reconstructions are one of the only ways to reconstruct leaf economic strategies in the fossil record, and the use of two-dimensional petiole width and leaf area measurements allows reconstructions to be done using common impression/compression leaf fossils. For DiLP, the incorporation of multiple continuous measurements that are functionally linked with climate improves the reproducibility of measurements and the accuracy of resulting climate reconstructions6,13. This protocol is designed to accommodate the incomplete nature of the fossil record by permitting leaf toothiness measurements to be made using leaf fragments. Although continuous measurements of leaf area provide more information about leaf size, DiLP MAP estimates can be complemented by those using leaf size classes in an effort to increase sample size16,53 or through the incorporation of vein scaling estimates of leaf area42,54,55. As with most involved methods, the time efficiency of this protocol will improve as a user becomes more experienced and confident, particularly in the preparation steps. The fact that the site-level DiLP measurements have been made following this protocol for >150 modern6,10,56 and at least 22 fossil assemblages to date attest to its feasibility6,38,39,40,41,42. Lastly, comprehensive measurements of leaf physiognomy have applications beyond those discussed here and may be useful in describing other aspects of plant ecology, physiology, evolution, and development, with application to both modern56 and paleo studies40.
In summary, the implementation of the methods detailed in this article allows a user to reconstruct paleoclimate and paleoecology using robust and reproducible methods. These methods provide an important opportunity to showcase past examples of climate and ecosystem responses to environmental perturbations and to provide further insight into the complex interactions of Earth's natural systems.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
AJL thanks the 2020-2022 undergraduate Team Leaf at the University of Washington for the motivation and suggestions for making effective training materials for DiLP. AGF, AB, DJP, and DLR thank the many undergraduates at Wesleyan University and Baylor University who measured modern and fossil leaves and whose input was invaluable in modifying and updating this protocol. The authors acknowledge the PBot Quantitative Traits Working Group and the PBOT team for encouraging the work to formalize this protocol to make it more accessible to broader communities. This work was supported by the National Science Foundation (grant EAR-0742363 to DLR, grant EAR-132552 to DJP) and Baylor University (Young Investigator Development Program to DJP). We thank two anonymous reviewers and the Review Editor for feedback that helped improve the clarity and comprehensiveness of this protocol.
Materials
| Copy stand or tripod | For fossil photography | ||
| Digital camera | For fossil photography, high resolution camera preferred | ||
| Image editing software | For digital preperation. Examples include Adobe Photoshop and GIMP, the latter of which is free (https://www.gimp.org/) | ||
| ImageJ software | IJ1.46pr | For making digital measurments, free software (https://imagej.net/ij/index.html) | |
| Microsoft Excel | Microsoft | Or similar software for data entry | |
| R software | The R foundation | For running provided R script (https://www.r-project.org/). R studio offers a user friendly R enviornment (https://posit.co/download/rstudio-desktop/). Both are free. | |
| dilp R Package | Can be installed following instructions here: https://github.com/mjbutrim/dilp |
Referenzen
- Gates, D. M. Transpiration and leaf temperature. Ann Rev Plant Physiol. 19 (1), 211-238 (1968).
- Givnish, T. On the adaptive significance of leaf form. Topics Plant Pop Biol. , 375-407 (1979).
- Niklas, K. J. . Plant biomechanics: an engineering approach to plant form and function. , (1992).
- Niklas, K. J. . Plant Allometry: The Scaling of Form and Process. , (1994).
- Royer, D. L., et al. Fossil leaf economics quantified: Calibration, Eocene case study, and implications. Paleobiology. 33 (4), 574-589 (2007).
- Peppe, D. J., et al. Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytol. 190 (3), 724-739 (2011).
- Wright, I. J., et al. The worldwide leaf economics spectrum. Nature. 428 (6985), 821-827 (2004).
- Royer, D. L., Miller, I. M., Peppe, D. J., Hickey, L. J. Leaf economic traits from fossils support a weedy habit for early angiosperms. Am J Botany. 97 (3), 438-445 (2010).
- Peppe, D. J., et al. Biomechanical and leaf-climate relationships: a comparison of ferns and seed plants. Am J Botany. 101 (2), 338-347 (2014).
- . dilp: Reconstruct paleoclimate and paleoecology with leaf physiognomy. R package version 1.1.0 Available from: https://cran.r-project.org/web/packages/dilp/index.html (2024)
- Bailey, I. W., Sinnott, E. W. A botanical index of Cretaceous and Tertiary climates. Science. 41 (1066), 831-834 (1915).
- Bailey, I. W., Sinnott, E. W. The climatic distribution of certain types of angiosperm leaves. Am J Botany. 3 (1), 24-39 (1916).
- Peppe, D. J., Baumgartner, A., Flynn, A. G., Blonder, B. Reconstructing paleoclimate and paleoecology using fossil leaves. Meth Paleoecol: Reconstructing Cenozoic Terre Environ Ecol Comm. , 289-317 (2018).
- Wolfe, J. A. . Temperature parameters of humid to mesic forests of eastern Asia and relation to forests of other regions of the Northern Hemisphere and Australasia. , (1979).
- Wilf, P. When are leaves good thermometers? A new case for Leaf Margin Analysis. Paleobiology. 23 (3), 373-390 (1997).
- Wilf, P., Wing, S. L., Greenwood, D. R., Greenwood, C. L. Using fossil leaves as paleoprecipitation indicators: An Eocene example. Geology. 26 (3), 203-206 (1998).
- Miller, I. M., Brandon, M. T., Hickey, L. J. Using leaf margin analysis to estimate the mid-Cretaceous (Albian) paleolatitude of the Baja BC block. Earth Planetary Sci Lett. 245 (1), 95-114 (2006).
- Wing, S. L., Greenwood, D. R. Fossils and fossil climate: The case for equable continental interiors in the Eocene. Philosophical Transact: Biol Sci. 341 (1297), 243-252 (1993).
- Wolfe, J. A. A method of obtaining climatic parameters from leaf assemblages. U S Geol Sur. (2040), 1-71 (1993).
- Huff, P. M., Wilf, P., Azumah, E. J. Digital future for paleoclimate estimation from fossil leaves? Preliminary results. Palaios. 18 (3), 266-274 (2003).
- Spicer, R. A. Recent and future developments of CLAMP: Building on the legacy of Jack A. Wolfe. Adv Angiosperm Paleobotany Paleoclimatic Reconstruct. 258, 109-118 (2007).
- Yang, J., Spicer, R. A., Spicer, T. E. V., Li, C. S. CLAMP Online’: a new web-based palaeoclimate tool and its application to the terrestrial Paleogene and Neogene of North America. Palaeobiodivers Palaeoenviron. 91 (3), 163-183 (2011).
- Little, S. A., Kembel, S. W., Wilf, P. Paleotemperature proxies from leaf fossils reinterpreted in light of evolutionary history. PLoS One. 5 (12), e15161 (2010).
- Webb, L. J. A physiognomic classification of Australian rain forests. J Ecol. 47 (3), 551-570 (1959).
- Schmerler, S. B., et al. Evolution of leaf form correlates with tropical-temperate transitions in Viburnum (Adoxaceae). Proc Royal Soci B: Biol Sci. 279 (1744), 3905-3913 (2012).
- Nicotra, A. B., et al. The evolution and functional significance of leaf shape in the angiosperms. Funct Plant Biol. 38 (7), 535-552 (2011).
- Leigh, A., Sevanto, S., Close, J. D., Nicotra, A. B. The influence of leaf size and shape on leaf thermal dynamics: does theory hold up under natural conditions. Plant Cell Environ. 40 (2), 237-248 (2017).
- Wright, I. J., et al. Global climatic drivers of leaf size. Science. 357 (6354), 917-921 (2017).
- Givnish, T. J. Leaf and canopy adaptations in tropical forests. Physiol Ecol Plants Wet Tropics. 12, 51-84 (1984).
- Feild, T. S., Sage, T. L., Czerniak, C., Iles, W. J. Hydathodal leaf teeth of Chloranthus japonicus (Chloranthaceae) prevent guttation-induced flooding of the mesophyll. Plant, Cell Environ. 28 (9), 1179-1190 (2005).
- Royer, D. L., Wilf, P. Why do toothed leaves correlate with cold climates? Gas exchange at leaf margins provides new insights into a classic paleotemperature proxy. Int J Plant Sci. 167 (1), 11-18 (2006).
- Edwards, E. J., Spriggs, E. L., Chatelet, D. S., Donoghue, M. J. Unpacking a century-old mystery: Winter buds and the latitudinal gradient in leaf form. Botanical Soc Am. 103 (6), 975-978 (2016).
- Givnish, T. J., Kriebel, R. Causes of ecological gradients in leaf margin entirety: Evaluating the roles of biomechanics, hydraulics, vein geometry, and bud packing. Am J Botany. 104 (3), 354-366 (2017).
- Royer, D. L., Wilf, P., Janesko, D. A., Kowalski, E. A., Dilcher, D. L. Correlations of climate and plant ecology to leaf size and shape: potential proxies for the fossil record. Am J Botany. 92 (7), 1141-1151 (2005).
- Green, W. A. Loosening the CLAMP: an exploratory graphical approach to the climate leaf analysis multivariate program. Palaeontol Electro. 9 (2), 19 (2006).
- Peppe, D. J., Royer, D. L., Wilf, P., Kowalski, E. A. Quantification of large uncertainties in fossil leaf paleoaltimetry. Tectonics. 29 (3), 002549 (2010).
- Royer, D. L., Meyerson, L. A., Robertson, K. M., Adams, J. M. Phenotypic plasticity of leaf shape along a temperature gradient in Acer rubrum. PLoS One. 4 (10), e7653 (2009).
- Lowe, A. J., et al. Plant community ecology and climate on an upland volcanic landscape during the early Eocene climatic optimum: McAbee fossil beds, British Columbia, Canada. Palaeogeograp, Palaeoclimatol, Palaeoecol. 511, 433-448 (2018).
- Flynn, A. G., Peppe, D. J. Early Paleocene tropical forest from the Ojo Alamo Sandstone, San Juan Basin, New Mexico, USA. Paleobiology. 45 (4), 612-635 (2019).
- Allen, S. E., Lowe, A. J., Peppe, D. J., Meyer, H. W. Paleoclimate and paleoecology of the latest Eocene Florissant flora of central Colorado, U.S.A. Palaeogeograp, Palaeoclimatol, Palaeoecol. 551, 109678 (2020).
- Baumgartner, A., Peppe, D. J. Paleoenvironmental changes in the Hiwegi Formation (lower Miocene) of Rusinga Island, Lake Victoria, Kenya. Palaeogeograp, Palaeoclimatol, Palaeoecol. 574, 110458 (2021).
- Wagner, J. D., Peppe, D. J., O’keefe, J. M., Denison, C. N. Plant community change across the Paleocene-Eocene boundary in the Gulf coastal plain, Central Texas. Palaios. 38 (10), 436-451 (2023).
- Ellis, B., et al. . Manual of leaf architecture. , (2009).
- Schneider, C. A., Rasband, W. S., Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 9, 671-675 (2012).
- Greenwood, D. R., Pigg, K. B., Basinger, J. F., DeVore, M. L. A review of paleobotanical studies of the Early Eocene Okanagan (Okanogan) Highlands floras of British Columbia, Canada, and Washington, USA. Canadian J Earth Sci. 53 (6), 548-564 (2016).
- Lowe, A. J., West, C. K., Greenwood, D. R. Volcaniclastic lithostratigraphy and paleoenvironment of the lower Eocene McAbee fossil beds, Kamloops Group, British Columbia, Canada. Canadian J Earth Sci. 55 (8), 923-934 (2018).
- Lowe, A. J., et al. Dynamics of deposition and fossil preservation at the early Eocene Okanagan Highlands of British Columbia, Canada: insights from organic geochemistry. Palaios. 37 (5), 185-200 (2022).
- Gushulak, C. A. C., West, C. K., Greenwood, D. R. Paleoclimate and precipitation seasonality of the Early Eocene McAbee megaflora, Kamloops Group, British Columbia. Canadian J Earth Sci. 53 (6), 591-604 (2016).
- Poorter, H., Niinemets, &. #. 2. 2. 0. ;., Poorter, L., Wright, I. J., Villar, R. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol. 182 (3), 565-588 (2009).
- Enquist, B. J., et al. Scaling from traits to ecosystems. Adv Ecol Res. 52, 249-318 (2015).
- Burnham, R. J., Wing, S. L., Parker, G. G. The reflection of deciduous forest communities in leaf litter: implications for autochthonous litter assemblages from the fossil record. Paleobiology. 18 (1), 30-49 (1992).
- Burnham, R. J., Ellis, B., Johnson, K. R. Modern tropical forest taphonomy: does high biodiversity affect paleoclimatic interpretations. PALAIOS. 20 (5), 439-451 (2005).
- Jacobs, B. F. Estimation of low-latitude paleoclimates using fossil angiosperm leaves: examples from the Miocene Tugen Hills, Kenya. Paleobiology. 28 (3), 399-421 (2002).
- Sack, L., et al. Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nat Comm. 3 (1), 837 (2012).
- Merkhofer, L., et al. Resolving Australian analogs for an Eocene Patagonian paleorainforest using leaf size and floristics. Am J Botany. 102 (7), 1160-1173 (2015).
- Baumgartner, A., Donahoo, M., Chitwood, D. H., Peppe, D. J. The influences of environmental change and development on leaf shape in Vitis. Am J Botany. 107 (4), 676-688 (2020).

