Using 2-Photon Microscopy to Quantify the Effects of Chronic Unilateral Ureteral Obstruction on Glomerular Processes
Summary
Here, we present a protocol using 2-photon microscopy in Munich Wistar Fromter rats with surface glomeruli to quantifythe effects of prolonged ureteral obstruction on glomerular dynamics and function.
Abstract
Applying novel microscopy methods to suitable animal disease models to explore the dynamic physiology of the kidney remains a challenge. Rats with surface glomeruli provide a unique opportunity to investigate physiological and pathophysiological processes using intravital 2-photon microscopy. Quantification of glomerular capillary blood flow and vasoconstriction and dilatation in response to drugs, permeability, and inflammation are just some of the processes that can be studied. In addition, transgenic rats, i.e., podocytes labeled with fluorescent dyes and other molecular biomarker approaches, provide increased resolution to directly monitor and quantify protein-protein interactions and the effects of specific molecular alterations.
In mice, which lack surface glomeruli after four weeks of age, unilateral ureteral obstruction (UUO) for several weeks has been used to induce surface glomeruli. As this induction model does not allow for baseline studies, we quantified the effects of UUO on glomerular processes in the UUO model in Munich Wistar Frömter (MWF) rats, which have surface glomeruli under physiologic conditions. The UUO model for five weeks or more induced significant alterations to gross renal morphology, the peritubular and glomerular microvasculature, as well as the structure and function of tubular epithelia. Glomerular and peritubular red blood cell (RBC) flow decreased significantly (p < 0.01), probably due to the significant increase in the adherence of white blood cells (WBCs) within glomerular and peritubular capillaries. The glomerular sieving coefficient of albumin increased from 0.015 ± 0.002 in untreated MWFs to 0.045 ± 0.05 in 5-week-old UUO MWF rats. Twelve weeks of UUO resulted in further increases in surface glomerular density and glomerular sieving coefficient (GSC) for albumin. Fluorescent albumin filtered across the glomeruli was not reabsorbed by the proximal tubules. These data suggest that using UUO to induce surface glomeruli limits the ability to study and interpret normal glomerular processes and disease alterations.
Introduction
Understanding glomerular processes, especially podocyte biology, has been a goal for over 50 years. Munich Wistar rats with surface glomeruli have played a central role in these studies, including micropuncture studies, to understand numerous aspects of physiologic and pathologic processes1,2,3. The utilization of microscopy to study glomerular components intravitally was limited due to the effects of phototoxicity until the advent of 2-photon microscopy that minimized this toxic exposure and increased the depth of penetration1,2. Along with rapid advancements in computer hardware and software, this has allowed for three-dimensional (3D) and four-dimensional (time) studies for hours in a single setting1,4,5.
The quantification of glomerular capillary blood flow, vasoconstriction and dilatation in response to drugs, permeability, and the effects of charge on permeability and inflammation are just some of the glomerular processes that have been studied. In addition, the S1 segment of the proximal tubule is identifiable, and the differences in the behavior of S1 and S2 tubular epithelium can be quantified1,4. Studies in mice, especially with the universal availability of mouse transgenic facilities, have led to rapid advances in the understanding of the molecular biology of glomerular disease processes. Individual proteins are responsible for glomerular dysfunction in knockout studies, especially with regard to proteinuria6,7,8. However, the utilization of mouse models for glomerular imaging studies has been limited as glomeruli are more than 100 µm below the surface in the numerous strains studied9.
This has led investigators to develop and utilize mouse models resulting in surface glomeruli that can be studied. The most common model is the use of complete UUO10,11,12. At the end of the extended UUO period, there are numerous surface glomeruli in mice kidneys that can be and have been studied13,14. There has been no baseline or control study in these mouse studies to determine the effects of prolonged UUO on glomerular biology. As this is a severe and prolonged model of injury resulting in rapid fibrosis and cortical destruction10,11,12, we hypothesized there would be effects on glomerular processes and function. To answer this question, Munich Wistar Fromter (MWF) rats with surface glomeruli were used to study control/baseline parameters, and the baseline finding was compared with glomerular studies in MWF rats following five weeks of UUO. We also studied Sprague Dawley (SD) rats that do not have surface glomeruli following UUO. The findings indicate that 5 weeks of UUO in MWF and SD rats do indeed increase the number of surface glomeruli. However, these were abnormal glomeruli with marked changes in glomerular blood flow, inflammation, and macromolecule permeability and size.
Protocol
All experiments followed the Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee at the Indiana University School of Medicine.
1. Preparing the Munich Wistar Frömter or SD rat for UUO surgery
- Anesthetize the rat using isofluorane (5% induction, 1.5-2.5% maintenance), then shave, wash, and disinfect the surgical area several times in a circular motion with both an iodine-based or chlorhexidine-based scrub and alcohol. Apply long-acting/slow-release analgesic for pain management as per the institutional IACUC guidelines.
- Make an incision along the midline using a scalpel; locate the left kidney and free it from the surrounding peritoneal organs.
- Carefully locate the renal pedicle, which comprises the renal artery, renal vein, and ureter. Separate the ureter from the other structures, taking precautions not to damage the delicate structure.
- Using fine forceps, carefully loop a 3-0 suture around the ureter and tie it off, being careful not to tear it. Repeat this procedure a few millimeters on either side of the first knot to tie a second knot and assure complete obstruction.
- Once the procedure is completed, carefully close the successive muscle layers. Prior to closing the final layer, add 2 mL of warm, sterile 0.9% saline into the abdomen before closing it completely. Close the outer skin with surgical staples.
- Apply long-acting/slow-release analgesic for pain management and observe recovery closely as per the institutional IACUC guidelines. Monitor periodically thereafter and prepare for imaging at the end of the fifth week.
2. Synthesis of Texas Red rat serum albumin (TR-RSA)
- Weigh out 100 mg of Rat Serum Albumin and dissolve it in 6.67 mL of 0.1 M sodium bicarbonate buffer at pH 8.4 in a 50 mL conical tube.
- To a vial of 5 mg of Texas Red-X-succinimidyl ester, add 100 µL of Dimethyl Formamide (DMF, high quality) and vortex until all the dye is dissolved.
- Place the rat serum albumin solution on a vortexer at a low/medium setting, so the solution volume is spinning well below the top of the open tube.
- Add the dissolved dye while the tube is being vortexed.
- Take the 50 mL conical tube, wrap it in foil, place the tube on any rocker or roller, and agitate slowly for 1 h at room temperature (RT).
- In a 5 L bucket of 0.9% NaCl with a stir bar under gentle stirring, wet the membranes of a suitable 50 kDa molecular weight cut-off dialyzer (a membrane with clips, enclosed membrane tubes, or dialysis cassettes are all suitable).
- Load the TR-RSA solution in the membrane system and attach it to the flotation attachments typically included with the system. Place the 5 L container with the 0.9% saline solution/TR-RSA overnight at 4 °C (in a cold room) with gentle agitation on a stir plate. Change the dialysis solution at least three times over the next 36 h.
- Swelling of the membrane will increase the volume of the now clear TR-RSA solution. Divide the original 100 mg by the volume to obtain an approximate concentration: the dye:protein ratio will be 1:1. Aliquot into suitable volumes and lyophilize for long-term storage.
3. Preparation for 2-photon intravital imaging on an inverted microscope
- Remove the cover of a 50 mm coverslip bottom dish (with 40 mm coverslip diameter) and place 8 pieces of autoclave tape on the inner bottom alongside the edge. Make a pyramid-shaped, empty window, using 4 pieces per side to allow the exteriorized kidney to fit snuggly in this space while maintaining contact peripherally with the autoclave tape, thereby helping to minimize motion. Adjust the spacing according to the size of the rat to assure the best contact with the kidney.
- Place 1 thermal pad on each side of the 50 mm coverslip bottom dish. Ensure the warming pad covers the stage.
- Use a 40x water immersion objective at 0.75x zoom and 1.5x zoom to generate 30x and 60x images, respectively, allowing for lower- and higher-magnification images. If necessary, add water to the objective using a 1 mL syringe with a long piece of PE-200 tubing that can reach the top of the objective at a downward angle to prevent wicking of the water droplet down the tubing.
- Use 2% laser transmissivity, with blue, green, and red detectors set to predetermined levels to assure consistency in the images between studies. Set the excitation wavelength to 800 nm on the referenced laser(see the Table of Materials), which will efficiently excite all the fluorophores used in this study.
- Use external (non-descanned) detectors to collect blue emissions using a photomultiplier tube (PMT) (420-490 nm, gain 950).
- Use a Hyd detector to capture green emissions (500-550 nm, gain 100).
- Use a Hyd detector to capture red emissions (590-660 nm, gain 200).
- Adjust the offset in the PMT (blue emissions) so that only a few pixels in the blank areas of tissue have a value of zero.
NOTE: The HyD detectors for the green and red emissions have automatic offset adjustment; only the gain can be set. - Set the bit-depth to 12-bit to give images a 4,096-intensity scale between black and white.
NOTE: It is necessary to set the lower limits of the detectors (offset in PMT) to not exclude these values to ensure collection of the low-intensity emissions within Bowman's space. If the sensitivity setting is too low, the visual warning markers will indicate this; these values are given an intensity value of zero.
- Dilute approximately 6 mg of Texas-Red-X-Rat Serum Albumin to a total volume of 1 mL, load the solution in a 1 mL syringe, and place on the indwelling i.v catheter in step 4.1 after infusion of Hoechst 33342 in step 9.1.
4. Surgical preparation for intravital 2-photon imaging
- Place the pre-anesthetized rat with an indwelling venous access line (femoral or jugular) on its side with the shaved left flank facing up flat and straight on the table. Ensure that the front paws touch each other, as should the rear paws.
- Gently palpate the left flank just below the ribs to feel for the kidney to determine the natural position in the abdomen. If needed, draw a line using a permanent marker along the shaved area, bisecting the kidney center in a nose-to-tail orientation.
- Using a pair of toothed forceps, grasp the skin and lift it upward to facilitate pinching of the permanent marker line with a pair of hemostats to crush the underlying vasculature and prevent bleeding when making the incision with a pair of surgical scissors. Repeat this for the thin outer muscle layer to minimize bleeding.
- To make the final incision of the thin inner abdominal muscle layer, repalpate the kidney to estimate the size and position. Carefully lift the inner muscle layer with a pair of forceps and crush a line that bisects the skin above the kidney with the hemostats that is approximately 1/3rd the estimated size of the kidney.
- Maintaining the grip on the muscle layer with the forceps, make the final incision.
NOTE: It is better to make a smaller incision and expand as needed than to make it too big, which will require partial closing with a suture. - Gently grip the kidney by the surrounding fat. Using both hands with forceps in each hand, work to grip the fat at the lower pole of the kidney by using a hand-over-hand technique to grip and hold the kidney fat, working downward.
- Having a firm grip on the fat at the lower pole of the kidney with one hand, gently pull the fat and, if needed, very gently squeeze the kidney through the incision. If the kidney does not pass through easily, widen the incision.
5. Positioning the rat for imaging
- Carefully place the exposed kidney against the edge of the dish, with a slight rotation so the abdominal side of the kidney is contacting the coverslip and the dorsal side is facing away from the edge.
- To further minimize motion, take two sterile 2 x 2 gauze pads, moisten them with saline, and pack them against the dorsal side of the kidney, reinforcing the contact of the abdominal side of the kidney to the edge.
- Look through the microscope eyepiece under epifluorescence illumination using a dual-pass Rhodamine/FITC cube. If motion is detected, make minor adjustments to the position and carefully adjust the gauze, making sure it does not push under the kidney. To further reduce motion, roll the rat over slightly, so the thorax is further away from the dish.
6. Image acquisition for quantitative analysis
- Scan the surface of the kidney using epifluorescence illumination (step 5.3) and mark the glomeruli position(s) using the software associated with the motorized stage controller (a feature of modern systems).
- For each color channel under 2-photon illumination, take a shallow 3D volume of the upper portion of each marked glomerulus, which will serve as the background images. Use a pseudocolor palette in the display option of the imaging software to better visualize the faint intensities of the background fluorescence of the glomerular capillary loops.
- Using a superficial blood vessel as the focal point, slowly infuse the fluorescent albumin, allowing time to observe the rise and fall of fluorescence due to systemic distribution. Infuse enough TR-RSA to achieve an intensity in the peritubular vasculature and capillary loops that is just below saturation.
NOTE: There is typically a 5 s delay between the infusion of material and its appearance in the bloodstream when renal perfusion is normal. - Wait approximately 10 min before acquiring 3D volumes (1 µm intervals) for all marked and imaged glomeruli from step 6.2.
NOTE: Simonsen's Munich Wistar rats have fewer surface glomeruli. However, as the Frömter strain of MW rats has a greater number of surface glomeruli, up to 10 glomeruli can often be imaged. - Euthanize the rat via an overdose of isoflurane at the end of the study. Perform a dual pneumothoracotamy to ensure euthanasia.
7. Calculating glomerular permeability
- Using the image viewing software associated with the microscope system, export the images to 12-bit raw images for processing and analysis.
- Load the background 3D volumes and the raw 3D volume containing the circulating fluorescent albumin. Locate the focal plane in the 3D volume with the brightest superficial capillary loop in the glomeruli with enough space to the edge of the surrounding Bowman's capsule.
- Using visual landmarks, locate the same focal plane found in the background volume. Select a region in the capillary loop and one within the Bowman's space noting the average intensity values of each one. Use these intensity values as background values.
- Outline a region (at least 20 x 20 pixels in area) within the Bowman's space in the albumin-containing image and note the intensity reading (select an area that is not adjacent to a capillary loop or the Bowman's capsule to assure the cleanest measurement of Bowman's space intensities). Move the drawn region over two other regions to take an average value for the average intensity within Bowman's Space.
- Select the brightest plasma fluorescent intensity within the capillary loop section and circle this region. Using the threshold function, highlight the bright values (usually located at the edges of the capillary loop walls), avoid circulating RBC shadows, and record the value.
NOTE: As factors within the blood will cause an underestimation of plasma fluorescence levels, it is important to select the brightest areas. - Enter the values in a spreadsheet to calculate the GSC using Eq (1):
GSC =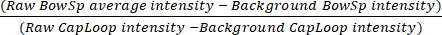 (1)
(1)
8. Calculating red blood cell flow in surface glomerular capillary loops and renal vasculature using a linescan function
- Find an appropriate vessel (either a capillary loop or a peritubular vessel). As the linescan function in the referenced image acquisition software (see the Table of Materials) requires the vessel to be perpendicular, rotate the image using the rotate function.
- Once the vessel is rotated and lying flat, select the XT function on the acquisition menu. Set up to scan 4,000 lines. Place the line across the vessel to be examined; ensure the focal plane is at the maximum diameter of the segment to be imaged.
- Left-click on the color composite image and select Take Snapshot to generate a reference image of the area where the linescan was taken. Immediately click the Start button to capture the linescan of the vessel.
- To determine the RBC flow rate, import the linescans into the image processing software (see the Table of Materials). Open the Show Region Statistics dialog box under the Measure dropdown menu. Select the single line drawing tool and draw a line that matches the slope of the RBC shadows. Note the width and height values.
NOTE: The pixel values obtained for width will correspond to distance; the pixels for height correspond to time. - Use the following formula (Eq (2)) to calculate speed.
RBC flow rate in µm/s =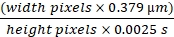 (2)
(2)
NOTE: This corresponds to acquisition parameters at 60x magnification and a 400 Hz scan rate with the referenced microscope (see the Table of Materials).- Make at least five calculations and average them to report the speed for each linescan.
NOTE: These parameters will be dependent on the pixel dimension and acquisition speed of the microscope system.
- Make at least five calculations and average them to report the speed for each linescan.
9. Calculating WBC occlusion in glomerular capillary loops
- Administer Hoechst 33342 nuclear stain (at ~8 µg/kg rat weight) via an indwelling venous access line to identify WBCs lodged in the capillary loops.
NOTE: The usable depth will be limited due to photon scatter and absorption by hemoglobin, especially for shorter wavelength blue emissions. - Center a glomerulus in the imaging field and take a 3D data set starting at the glomerular surface and end the data at least 30 to 35 µm. Use a 1 µm step size in the Z-direction.
- Identify WBCs by comparing the blue Hoechst channel with the Texas Red albumin channel; look for exclusion of red dye in the capillary loop and a corresponding nuclear stain to positively identify the WBCs. Define white blood cells as "adhered" if they appear static over 3 optical sections. Report the values as occurrence/10 optical sections from the top of the glomerulus, taken at 1 µm intervals.
10. Scoring the presence of Rouleaux formations in surface glomeruli
- Follow the same directions for step 9.3 and acquire a 3D data set. Look for Rouleaux formations appearing as RBCs stacked into packets that resist dissociation as they move along the capillary loops. Use a red fluorophore for better visualization of structure at greater depths due to less photon scatter for the longer wavelength red emissions. Report the values as occurrence/25 optical sections from the top of the glomerulus, taken at 1 µm intervals.
11. Isolation of glomeruli
- Isolate three groups of glomeruli from fresh kidneys using a standard sieving technique that results in close to 90% purity of rat glomeruli15.
- Place the kidney cortex in cold phosphate-buffered saline (PBS) and mince it using multiple fine scissors or razor blades.
- Add the minced tissue to a 100 µm sterile cell strainer and gently push it through using a 5 mL syringe plunger and 50-100 mL of cold PBS.
NOTE: Most of the tubules are retained while the glomeruli pass through. - Place the enriched glomeruli fraction onto a 70 µm filter and wash them extensively with cold PBS. Wash the filter with 100-200 mL of cold PBS to remove most of the remaining tubules.
- Collect the glomeruli from the filter using 1-2 mL of cold PBS, centrifuge (10,000 × g, 2 min, 4 °C) and snap-freeze them in liquid nitrogen until RNA isolation.
NOTE: Glomerular purity determined by phase-contrast microscopy is >90%, and the yield is approximately 10 mg from 2 kidneys.
12. Glomerular RNA isolation
- Homogenize the frozen glomeruli pellet using the RNA isolation reagent by adding 400 µL of the reagent and breaking up the pellet using a 200 µL pipet tip, followed by brief vortexing and 5 min of incubation at RT16.
- Add 40 µL of 1-bromo-3-chloropropane (BCP), vortex for 15 s, and hold at RT for 15 min.
- Centrifuge at 12,000 × g, 15 min, 4 °C. Remove the aqueous layer, dilute the lower layer with an equal volume of 70% ethanol, and load it directly onto a spin column (see the Table of Materials).
- After the washes, each consisting of adding the respective solution to the column followed by centrifugation at 12,000 x g, 15 s, 26 °C [3 total, first 2 with 500 µL of RPE (proprietary mild washing buffer with ethanol to remove traces of salts, 3rd wash with 500 µL of 80% EtOH], elute the RNA by the addition of 15 µL of H2O and centrifuge, as for the washes. Check the concentration and purity of the RNA and transport the RNA samples to the core facility for Nanostring analysis17,18.
NOTE: Total RNA yield is approximately 1-2 µg. Here, the 24 samples contained 200 ng of RNA at 30 ng/µL.
13. Nanostring analysis
NOTE: Nanostring technology is based on the digital detection and direct molecular barcoding of target molecules that utilize color-coded probe pairs. The Capture probe carries a biotin moiety on the 3' end, and the Reporter probe carries the signal on its 5' end.
- Ship the Nanostring gene probe pairs and CodeSets to Michigan State's Genomic Core Facility and use as directed by NanoString.
NOTE: There are six positions for the color codes, and each position can be one of four colors, enabling a large diversity of tags that can each be individually resolved and identified during data collection. - Obtain the data collected by the Genomic Core facility staff using the proprietary Nanostring nCounter Digital analyzer, which collects fields of view using a microscope objective and CCD camera and tabulates and displays the barcode counts.
- Import the raw data into Nanostring's nSolver software for analysis. Normalize the data normalized using their default settings and compare data between groups as outlined in their manual.
NOTE: The objective was to monitor gene changes previously shown to be altered in cortical tissue from a UUO model19, kidney disease17,20,21,22,23,24,25,26. A total of 126 genes, including the recommended positive and negative controls, were analyzed in each glomeruli group (CONT, SHAM, & UUO).
Representative Results
Three groups of glomeruli were isolated using a standard sieving technique that results in close to 90% purity of rat glomeruli15. The first glomeruli group was from the left kidney of SD rats that underwent a left kidney ureter clamp for 5 weeks, UUO (5 males, 3 females). The second glomeruli group was isolated from the contralateral control kidney from the same rat, CONT (5 males, 3 females). The third group of glomeruli was isolated from SD rats that underwent SHAM surgery, and the left kidney was used for glomeruli isolation after 5 weeks, SHAM (4 males, 4 females).
Morphological alterations
Externalization of the obstructed kidney for imaging revealed a grossly enlarged kidney, approximately four times the normal size, with thin epithelia visible through the fluid-filled kidney interior. Through a 20x objective, using epifluorescence illumination and a FITC/Rhodamine dual-pass cube, the most apparent change was the thinning of the tubular epithelia and the uniform collapse of tubular lumens along the entire tubular length. The histology has been well described and is severe by one week of UUO10,11,12. The number of glomeruli visible at the surface in MWF and SD rats increased after five weeks of unilateral ureter obstruction. Figure 1A shows the method used to create the UUO model. The right kidney is untouched and provides an adequate glomerular filtration rate for the rat. The number of glomeruli per field using a 20x objective (363 µm x 363 µm) was counted and shown in a graph in Figure 1B. The number of surface glomeruli in MWF rats increased from 1.08 ± 0.11/field in untreated rats to 2.97 ± 0.65/field in the five-week UUO group. SD rats went from having no surface glomeruli to 2.02 ± 0.37/field following 5 weeks of UUO.
Intravital 2-photon images were taken of these rats after injection with TR-RSA (red), a 10 kDa Cascade Blue dextran (10 kDa-CB), and Hoechst 33342 to label the nuclei (cyan). These are shown for normal MWF rats (Figure 1C), MWF rats after five weeks of UUO (Figure 1D), and SD rats after five weeks of UUO (Figure 1E). These images highlight dramatic alterations that occur in the tubular epithelia. Proximal tubule lysosomes, which are normally small punctate orange-colored accumulations in untreated MWF and SD rats, become large singular vacuolar structures that fill the majority of the shrunken tubular cell. As outlined by the TR-RSA albumin, the vasculature appeared straightened, and in many vessels, devoid of flowing RBCs, showing only streaming plasma. Fixation of the kidneys revealed that the cortex had thinned out into a fibrous skin no thicker than a millimeter. These observations agree with early literature using this model3,10,11,12.
Alterations in renal vascular dynamics and glomerular permeability
Renal blood flow was significantly reduced in both the five-week UUO MWF and SD groups compared to untreated rats (Figure 2). Sham-operated MWF rats had a peritubular RBC flow rate of 885 ± 25 µm/s. Peritubular RBC flow in five-week UUO MWF and SD rats decreased to 250 ± 100 µm/s and 200 ± 125 µm/s, respectively. These values were calculated by collecting line scans across peritubular vessels to calculate RBC speed. Figure 2D shows a graph of these data.
The RBC speed within the glomerular capillary loops was significantly decreased in the five-week UUO MWF and SD rats compared to untreated MWF (Figure 3). In many cases, glomeruli were found to have capillary loops completely devoid of flowing RBCs. Capillary loop RBC flow rates were 1,405 ± 425 µm/s, 250 ± 220 µm/s ±, and 190 ± 200 µm/s or untreated MWF, five-week UUO MWF, and five-week UUO SD rats, respectively (Figure 3D). Within the capillary loops of the five-week UUO groups, the sluggish flow of the RBCs revealed the presence of adherent WBCs, either slowing flow or blocking it, with only plasma flow visualized downstream from the partial or total obstruction. To quantify this observation, the number of adherent WBCs found in a 3D volume was counted and then normalized to occurrence per every 10 µm of 3D volume depth. The structure of the adhered white cell could be discerned using the cyan nuclear dye fluorescence from Hoechst 33342. Unfortunately, the greater photon scatter of blue-emitting lights limited reliable identification of WBCs by their nuclei to the upper 10 optical sections from the top, taken at 1µm steps of glomerular volume. Untreated MWF rats had less than 0.125 ± 0.05 WBCs/ 10 optical sections from the top, taken at 1 µm steps volume while this number increased to 1.5 ± 0.5 and 3.25 ± 0.7 in 5-week UUO MWF and 5-week UUO SD rats, respectively (Figure 3E).
Another vascular alteration that may also account for reduced RBC flow in the 5-week UUO groups was the regular appearance of Rouleaux formations (grouped RBCs that are adhered in a "stacked coin" configuration, see inset of Figure 3F). Rouleaux formations flow more slowly and can be stopped by an adherent WBC. Untreated WMF rats have virtually no Rouleaux formations in their glomerular capillaries, having only 0.05 ± 0.05 occurrences per 25 optical sections from the top, taken at 1 µm steps . The five-week UUO MWF and SD rats had a marked increase in Rouleaux formations 2.27 ± 0.46 and 1.46 ± 0.73 per 25 optical sections starting from the top, taken at 1 µm steps, respectively (Figure 3F).
In addition to the reduction in glomerular RBC flow rates seen with UUO, an increase in albumin permeability was seen. There was greater heterogeneity in albumin permeability between glomeruli. Occasionally, albumin accumulation within the Bowman's Space was intense enough to be clearly seen (Figure 4B, asterisk). The glomerular sieving coefficient of albumin increased from 0.015 ± 0.002 in untreated MWFs, to 0.045 ± 0.05 in 5-week UUO MWF and 0.052 ± 0.075 in five-week UUO SD rats.
Altered proximal tubule function
Interestingly, the filtered albumin could not be detected in proximal tubule cells following UUO. The S1 segment normally endocytoses large quantities of albumin27,28,29,30, as shown here under physiologic conditions in untreated MWF rats (Figure 5A). This same uptake could not be seen in the MWF or SD PT following 5 weeks of UUO (Figure 5B,C). Proximal tubules surrounding the glomeruli imaged between 45 and 60 min post TR-RSA infusion were scored for either presence (1) or absence (0) of albumin. It is important to note that under physiologic conditions, the S1 segment avidly binds and internalizes albumin with little to no albumin reaching the distal tubules or collecting ducts. Therefore, it stands to reason that the latter proximal tubule segments may not contain albumin resulting in a fractional positivity for albumin uptake of less than 1.0. Figure 5D shows a graph with the results from scoring proximal tubule albumin uptake. Untreated MWF had a proximal tubule fractional uptake value of 0.556 ± 0.126. Both five-week UUO MWF and SD rats had significantly lower values of 0.049 ± 0.126 and 0.00 ±0.00, respectively.
Studies were also completed in 12-week UUO MWF rats (Table 1). Twelve weeks of UUO is the standard time used for mouse studies to induce surface glomeruli. Three UUO male rats were imaged, and the glomerular density increased further to 6.16 ± 1.83 glomeruli per 20x field in these rats. The RBC flow rate was 293 ± 67 µm/s, WBC adhesion was 1.47 ± 1.12, both similar to the 5-week UUO data. The GSC of albumin also increased compared to the 5-week UUO rats to 0.109 ± 0.04.
Glomerular mRNA alterations induced by the chronic UUO
Table 2 shows all genes (probes) with their grouped expression and standard deviation values. Note, genes were selected for analysis based on previous documentation of alteration in kidney disease, including UUO as described in protocol section 13's note. Figure 6 is a heat map of the data highlighting the dramatic changes in gene expression for most genes in the UUO glomeruli compared to either control or sham glomeruli.

Figure 1: Increase in the number of surface glomeruli and induction of surface glomeruli in SD rats following 5-week UUO in MWF rats. (A) A surgical diagram of the ureter on the left kidney carefully freed from the renal artery and vein prior to being occluded with two ties using surgical suture. (B) A graph indicating the increase in the number of surface glomeruli present in MWF rats prior to and five weeks post UUO along with the number of surface glomeruli in SD rats, which normally have no surface glomeruli. The number of surface glomeruli in MWF rats increased from 1.08 ± 0.11/field in untreated rats to 2.97 ± 0.65/field in the 5-week UUO group. SD rats went from having no surface glomeruli to 2.02 ± 0.37/field. Three-dimensional reconstructed images show the renal surface for untreated MWF (C), MWF after 5-week UUO (D), and SD after five-week UUO (E). Note the appearance of large, orange vacuolar structures that have coalesced from small individual lysosomes into large abnormal bodies. The 5-week UUO in SD rats did not result in areas resembling normal tubular epithelia seen in C and partially in D. The vasculature appeared straightened in some regions and, in many, had partial occlusions that allowed plasma but not RBCs to flow. (n =3 male rats per group) Scale bars = 40 µm. Error bars indicate standard deviation. Abbreviations: UUO = unilateral ureteral obstruction; SD = Sprague-Dawley; MWF = Munich Wistar Frömter; G = glomerulus; RBCs = red blood cells. Please click here to view a larger version of this figure.
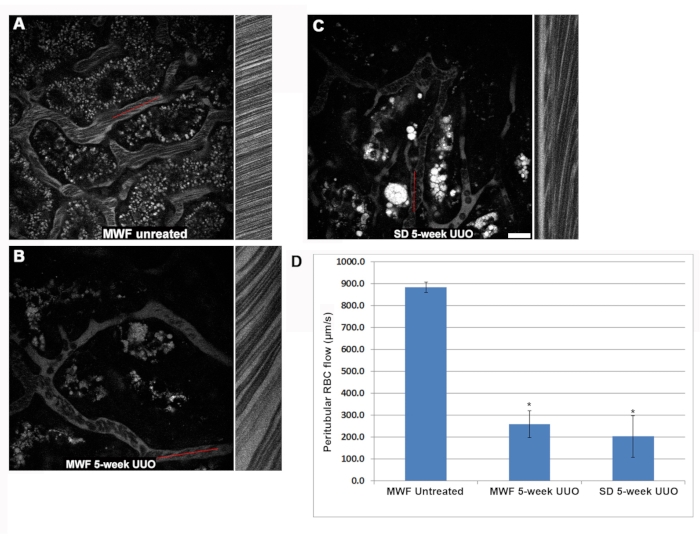
Figure 2: Reduction in RBC flow within the superficial peritubular vasculature after 5-week UUO. Linescans were collected in peritubular blood vessels to determine RBC flow speed in µm/s. Briefly, the red reference lines shown in A, B, and C represent a small region in which the same pixel wide area was scanned repeatedly, and the images stacked in a column to visualize the distortion caused by the flowing RBCs, which travel faster than the microscope can acquire them. The column adjacent to the reference figure is the linescan, with the slope of the RBC distortion being used to calculate speed (x-axis = distance and y-axis = time). Here, progressively steeper slopes correspond to slower RBC speeds as they remain longer in the linescan region. Note the difference in the appearance of the RBCs in untreated MWF rats (A) as compared to the five-week UUO images for the MWF (B) and SD (C) rats. The RBC flow speeds for the three rat groups are shown in D. Peritubular RBC flow in untreated MWFs averaged 885 ± µm/s. These values dropped significantly five weeks post UUO in MWF and SD rats to 250 ± 100 µm/s and 200 ± 125 µm/s, respectively. Scale bar = 20 µm, n = 3 male rats per group. Error bars indicate standard deviation. Abbreviations: UUO = unilateral ureteral obstruction; SD = Sprague-Dawley; MWF = Munich Wistar Frömter; RBC = red blood cell; WBC = white blood cell. Please click here to view a larger version of this figure.
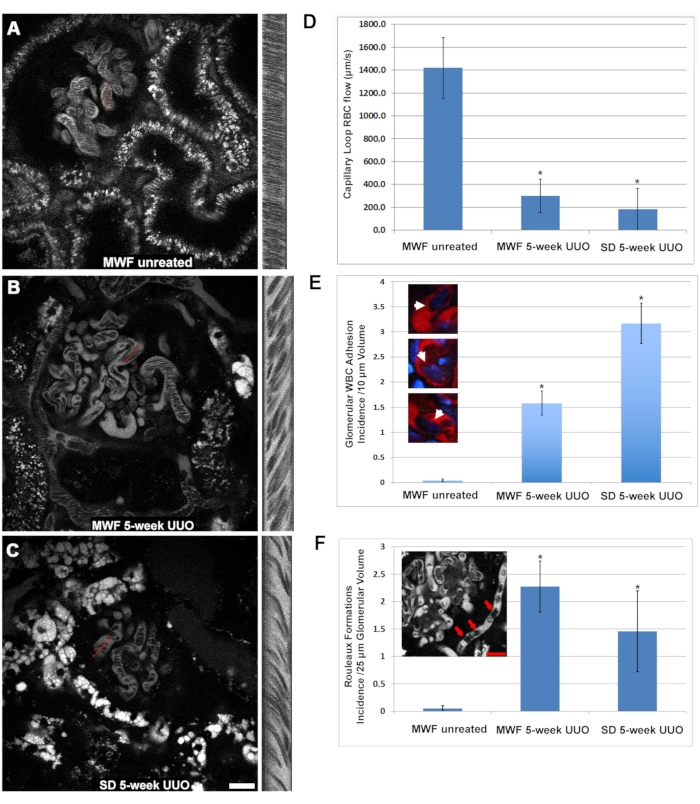
Figure 3: Significant reduction in glomerular capillary loop RBC flow and induced activation of WBC adhesion by 5-week UUO. The same linescan approach used to determine peritubular RBC flow was used to investigate alterations in glomerular capillary blood flow. Panels A, B, and C show a similar layout to Figure 2 and focus on a glomerulus in sham-operated MWF, five-week UUO MWF, and five-week UUO SDs, respectively. (D) A graph revealing the physiologically high RBC flow rate in the capillary loops from sham-operated MWF rats averaging 1,405 ± 425 µm/s ± 425, decreased to 250 ± 220 µm/s and 190 ± 200 µm/s for the5-week UUO MWF and 5-week UUO SD rats, respectively. When examining the glomerular capillary loops, adherent WBCs were readily visible while focusing through the glomerulus. Three-dimensional optical sections of individual glomeruli were taken, and the first 10 optical sections, 1 µm apart, were used to measure the number of adherent WBCs. Untreated MWF rats had virtually no visible WBCs in their volumes, averaging less than 0.125 ± 0.05 WBCs/10 optical sections from the top, taken at 1µm steps. In 5-week UUO MWF and SD rats, these numbers increased to 1.5 ± 0.5 and 3.25 ± 0.7 WBCs/10 optical sections from the top, taken at 1 µm steps, respectively. These results are shown in the graph in panel E, with color inserts showing WBCs occluding capillary loops. Rouleaux formations (arrows, inset in panel F) appear as RBCs tightly bound together in a "stacked coin" configuration which largely retain their bundled grouping even in the turbulence of blood flow. These pathologic structures were easily discernible at greater depths within the glomerulus. Sham-operated MWF rats were largely devoid of these structures, having only 0.05 ± 0.05 occurrences 25 optical sections from the top, taken at 1 µm steps of glomerular volume. In contrast, the 5-week UUO in both MWF and SD rats had a marked increase in Rouleaux formations with occurrences of 2.27± 0.46 and 1.46 ± 0.73/25 optical sections from the top, taken at 1 µm steps, respectively. Scale bars= 20 µm, n = 3 male rats per group. Error bars indicate standard deviation. Abbreviations: UUO = unilateral ureteral obstruction; SD = Sprague-Dawley; MWF = Munich Wistar Frömter; RBC = red blood cell; WBC = white blood cell. Please click here to view a larger version of this figure.
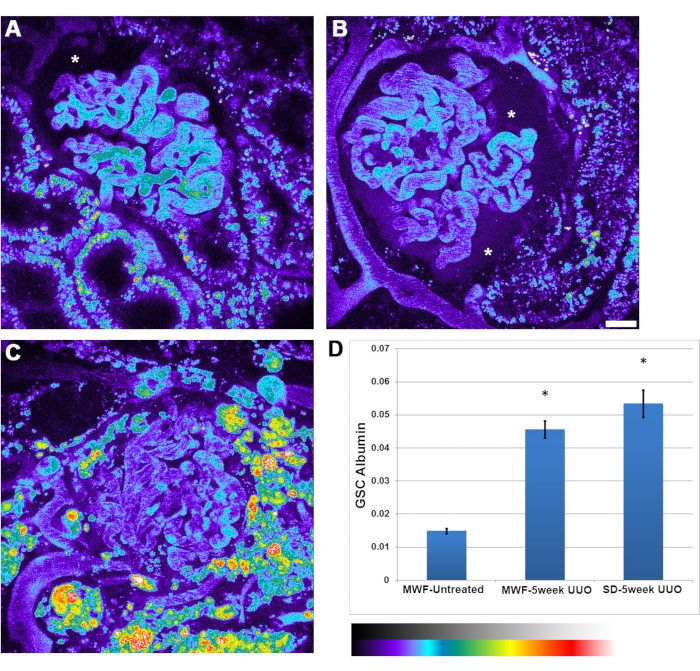
Figure 4: Significant increase in glomerular capillary albumin permeability following 5-week UUO. Panels A, B, and C show pseudocolor images of a 3D volume for rat serum albumin channel in untreated MWF, 5-week UUO MWF, and five-week UUO SD rats, respectively. The images are presented in a pseudocolor palette to highlight the appreciable amount of filtered albumin seen in the Bowman's space, particularly in panel B (asterisk). (A) Bowman's space (asterisk) shows the normal level of albumin typically seen in untreated MWF rats, indiscernible to the eye. Images of glomeruli were taken prior to the infusion of albumin to subtract background fluorescence values from those taken after albumin administration. (D) A graph with the glomerular sieving coefficient for albumin in untreated MWF rats, having a value of 0.015 ± 0.002. This value increased significantly to 0.045± 0.05 in 5-week UUO MWF rats and 0.052 ± 0.075 in five-week UUO SD rats. This parameter is a ratioed value of fluorescent intensities of Bowman's Space divided by the plasma value and has no associated unit of measure. Scale bar = 20 µm, n = 3 male rats per group. Pseudocolor intensity scale is located below panel D. Error bars indicate standard deviation. Abbreviations: UUO = unilateral ureteral obstruction; SD = Sprague-Dawley; MWF = Munich Wistar Frömter; RBC = red blood cell; WBC = white blood cell. Please click here to view a larger version of this figure.
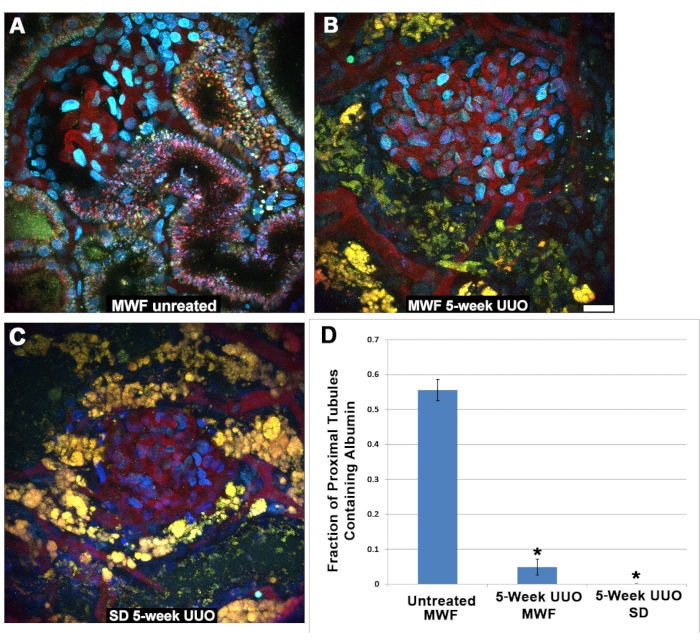
Figure 5: Reduced function in proximal tubules following 5-week UUO. (A) An image of a superficial glomerulus and the S1 segment from a normal Munich Wistar Frömter rat taken 40 min after infusion of TR-RSA and Cascade Blue dextran. Internalization of the TR-RSA can be seen in the S1 segment and the proximal tubule. In sharp contrast, MWF rats (B) and SD rats (C), subjected to 5-week UUO, display severely altered proximal tubules with minimal or no uptake of TR-RSA. The normally small, punctate autofluorescent lysosomes (A) become large and vacuolar yellow-orange structures, often with a complete collapse of the tubular lumen, which cannot be found in the three-dimensional data sets. (C) Distal tubules normally devoid of any form of autofluorescence now contain autofluorescent accumulations. Scoring the proximal tubules surrounding the glomeruli in similar images taken 45-60 min following infusion, for albumin uptake, showed a significant difference between the sham-operated MWF group and both 5-week UUO groups. Untreated MWF rats had a proximal tubule fractional uptake value of 0.556 ± 0.126. Both five-week UUO MWF and SD rats had significantly lower values of 0.049 ± 0.126 and 0.00 ±0.00, respectively. Scale bar= 20 µm, n = 3 male rats per group. Error bars indicate standard deviation. Abbreviations: UUO = unilateral ureteral obstruction; SD = Sprague-Dawley; MWF = Munich Wistar Frömter; RBC = red blood cell; WBC = white blood cell; TR-RSA = Texas Red rat serum albumin. Please click here to view a larger version of this figure.
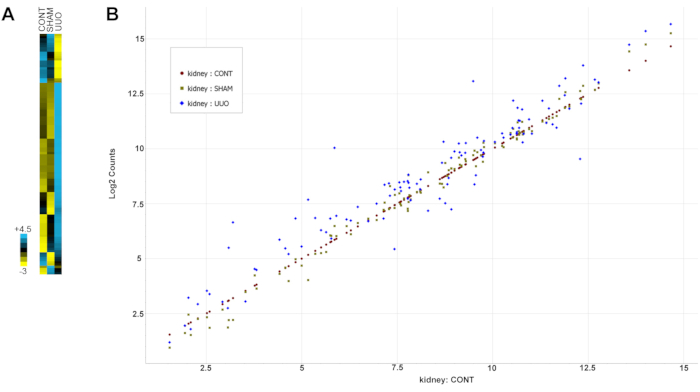
Figure 6: Gene expression alterations with UUO. (A) A heat map of the data. (B) Normalized gene changes for all data in a scatter plot. Expression of genes in the control kidneys was plotted from low to high expression, and the genes from both SHAM and UUO kidneys were compared to the control expression levels. Gene data points falling close to the control genes diagonal value indicate similar expression levels for both groups, while data points above or below the diagonal indicate higher or lower expression levels, respectively. Note the SHAM genes cluster closer to the control diagonal expression than the UUO genes, which are more variable. Abbreviation: UUO = unilateral ureteral obstruction. Please click here to view a larger version of this figure.
Table 1: Progression of injury from 5 to 12 weeks of UUO. Comparison of 5- and 12-week UUO imaging parameters in three male MWF rats and three SD male rats at each time point. Five-week data are the same as in the previous figures. Note the increase in glomerular density and the GSC for albumin with continued UUO. Abbreviations: UUO = unilateral ureteral obstruction; SD = Sprague-Dawley; MWF = Munich Wistar Frömter; GSC = glomerular sieving coefficient. Please click here to download this Table.
Table 2: Analysis of inflammation markers in glomeruli from UUO and control rats. Gene probe pairs and CodeSets were designed and used as directed by NanoString. Over 100 genes were analyzed, plus positive and negative controls as specified by Nanostring. All genes (probes) with their grouped expression and SD values are shown in the table. Figure 6A is a heat map of the data, while Figure 6B presents the gene changes for all data in a scatter plot. Note the similarities between controls and SHAM and distinct changes for most genes in the UUO. Abbreviations: UUO = unilateral ureteral obstruction; SD = standard deviation. Please click here to download this Table.
Discussion
The study of glomerular physiology has seen many different approaches, most notably the use of micropuncture, perfusion of isolated glomeruli, and microscopy. The availability of surface glomeruli in Munich Wistar rats, Fromter and Simonsen strains, has allowed in vivo dynamic studies. One important note to investigators adopting this technology is the need to set acquisition parameters to maintain consistent images between studies, so the autofluorescence in tissue remains consistent. Utilizing a dual-pass fluorescein/rhodamine epifluorescence cube and adjusting gain settings to the green and red emission channels to mimic on the computer screen what is seen through the eyepieces will ensure a consistent color signature in the autofluorescence even between different microscope systems.
The Fromter strain has been used extensively as it has a reduced number of total glomeruli, ~75% normal, and the males spontaneously develop hypertension at around 12 weeks of age, with progressive proteinuria and subsequent focal glomerular sclerosis, eventually dying of kidney failure12. The use of these rats and the addition of 2-photon microscopy with its reduced phototoxicity, improved depth of penetration, and the ability to view multiple fluorescent probes simultaneously paved the way for new discoveries1,4,5. With the development of computer hardware and software, quantitative data are now the standard for all 2-photon laboratories. Multiple quantitative techniques have been developed and applied to glomerular, proximal tubule, vascular, and interstitial processes under physiologic and disease conditions1,4,5,27,28,29,30.
Transgenic mouse generating facilities added a new dimension to the study of kidney physiology and pathology, and it was only a matter of time until this was combined with 2-photon microscopy to further delineate the importance of specific gene products in kidney structure and function. However, mouse glomeruli, except in very young mice, are located over 100 µm from the surface of the kidney9. Two-photon microscopy is best undertaken at a depth of between 20 and 50 µm as resolution, and fluorescence intensity diminishes rapidly thereafter due to light scattering of emitted light and absorption from interaction with hemoglobin. Therefore, it was necessary to induce surface glomeruli. The approach commonly used is a prolonged unilateral obstruction model for 12 weeks. As these models do not allow baseline determinations, separation of the effects of UUO from the process being studied is not possible.
Using MWF rats, one can compare baseline glomerular function with that following UUO. This UUO model is known to induce inflammation and a rapid rate of fibrosis and has been used to study CKD and fibrosis10,11,12. As expected, there was an increase in surface glomeruli in both the MWF and SD rats. Moreover, the quantitative results obtained following UUO for the MWF and SD rats were very comparable. The reduction in blood flow recorded here had been previously reported comparing microscopic data following UUO to micropuncture data3. It was also well known that tubular and interstitial histology is markedly altered, and the PTs are mostly nonfunctional, as reported here, with a lack of albumin endocytosis. The studies in Figure 2 and Figure 3 show a dramatic reduction in RBC flow rate in glomerular and peritubular capillaries and enhanced WBC adhesion. The reductions in flow are likely due to capillary blockage from WBC adhesion and rouleaux formations.
To further evaluate inflammation, we quantified albumin permeability and showed it to increase tenfold. Additionally, isolated glomeruli showed that mRNA expression increased for many genes previously known to be increased in kidney inflammation in a variety of kidney disease states17,19,20,21,22,23,24,25,26. The increases in glomerular surface density and albumin permeability were progressive, as shown by the 12-week UUO data. The present data are the first to directly show that glomeruli undergo significant structural damage, inflammation, and molecular changes in the UUO model. The results are consistent with an earlier study of whole kidney tissue that analyzed sheep kidney biopsies following UUO, finding multiple inflammation markers elevated19. The present results indicate marked inflammation exists within the glomeruli, previously only known for cortical tissue.
The present data differs from earlier studies in mice where no changes were found in adhesion molecule expression, complement deposition, and neutrophil infiltration between 12-week posthydronephrotic and normal glomeruli31. In addition, the Hickey laboratory used the 12-week UUO model to study immune reactions in glomeruli of mice. They found no differences in neutrophil infiltration between four-week-old mouse glomeruli and postobstructive glomeruli32,33. These later studies were conducted after the pelvis of the obstructed kidney was drained of urine. We did not do this as we wanted to determine the effect of UUO on glomerular function as it would be in vivo, without artificially removing the fluid causing the obstruction. Finally, the use of UUO in mice is being replaced by imaging glomeruli at greater than 100 µm beneath the surface. While possible, there is a trade-off of in resolution and intensity, both being reduced significantly as one goes beyond 50 µm34.
The results presented are not surprising if one pieces together the data from the existing literature on histologic changes, formation of atubular glomeruli, inflammation, fibrosis, hemodynamics10,11,12. The data presented, including WBC adhesion, rouleaux formations, glomerular molecular inflammation markers, and increased albumin permeability, further indicate the extensive inflammation that is ongoing in this UUO model even at five weeks and also present at twelve weeks. Clearly, chronic UUO is not a physiologic state, and the use of UUO to induce surface glomeruli represents an injury model. The MWF rats, which have surface glomeruli under physiological conditions, can be longitudinally studied as injury occurs. It is possible to generate transgenic rats, and numerous investigators are creating them with biosensors to ask specific questions. In particular, the Medical College of Wisconsin now has a colony of MWF rats and has made transgenic rats for the purpose of studying glomerular processes under physiologic and pathologic conditions. These MWF rats offer a great opportunity to study glomerular processes in normal, diseased, and genetically altered rats.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1DK091623 and P30DK079312 (to B.A.M.). We thank the staff at the Genomics Core Facility of the Research Technology Support Facility (RTSF) at Michigan State University for performing the Nanostring analysis.
Materials
| 70 µm sterile cell strainer | Corning | #421751 | |
| 100 µm sterile cell strainer | Corning | #421752 | |
| CA Micro scissors Model 1C300 | Electron Microscopy Sciences | Cat# 72930 | |
| Electric heating pad | Sunbeam | Kroger | |
| Handling Forceps | Electron Microscopy Sciences | Cat# 72962 | |
| Kelly Hemostatic Forceps (straight) | Electron Microscopy Sciences | Cat#72930 | |
| Leica Dive SP-8 Multi-Photon Inverted Microscope | Leica Microsystems | Note: Version 7.1r1 | |
| MaiTai DeepSee titanium-sapphire laser | Spectra-Physics | NA | |
| Mayo Dissecting Scissors | Electron Microscopy Sciences | Cat# 78180-1C3 | |
| Metamorph Image processing Software | Molecular Dynamics | Cat# 78266-04 | |
| Microsoft Excel | Microsoft Corportation | 2007 version | |
| Quant-iT RNA Assay Kit | Invitrogen/ThermoFisher | Q33140 | |
| Reptitherm Undertank Heater | Zoomed | Amazon | |
| RNeasy MinElute Cleanup Kit (Spin columns) | Qiagen | 74204 | |
| RPE buffer | Qiagen | 1018013 | |
| Strate-Line Autoclave Tape | Fisher Scientific | Cat# 11-889-1 | |
| TRI Reagent | Sigma | T9424 | |
| Willco-dish Coverslip Bottom Dishes (50 mm/40 mm coverslip) | Electron Microscopy Sciences | Cat# 70665-07 |
Referenzen
- Dunn, K. W., Molitoris, B. A., Dagher, P. C. The Indiana O’Brien center for advanced renal microscopic analysis. American Journal of Physiology-Renal Physiology. 320 (5), 671-682 (2021).
- Dunn, K. W., et al. Functional studies of the kidney of living animals using multicolor two-photon microscopy. American Journal of Physiology-Cell Physiology. 283 (3), 905-916 (2002).
- Eisenbach, G., Liew, J., Boylan, J., Manz, N., Muir, P. Effect of angiotensin on the filtration of protein in the rat kidney: a micropuncture study. Kidney International. 8 (2), 80-87 (1975).
- Sandoval, R. M., Molitoris, B. A. Intravital multiphoton microscopy as a tool for studying renal physiology and pathophysiology. Methods. 128, 20-32 (2017).
- Sandoval, R. M., Molitoris, B. A., Palygin, O. Fluorescent imaging and microscopy for dynamic processes in rats. Methods in Molecular Biology. 2018, 151-175 (2019).
- Huber, T., et al. Molecular basis of the functional podocin-nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Human Molecular Genetics. 12 (24), 3397-3405 (2003).
- Kawachi, H., Koike, H., Kurihara, H., Sakai, T., Shimizu, F. Cloning of rat homologue of podocin: expression in proteinuric states and in developing glomeruli. Journal of the American Society of Nephrology JASN. 14 (1), 46-56 (2003).
- Roselli, S., et al. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Molecular and Cellular Biology. 24 (2), 550-560 (2004).
- Schießl, I., Bardehle, S., Castrop, H. Superficial nephrons in BALB/c and C57BL/6 mice facilitate in vivo multiphoton microscopy of the kidney. PloS One. 8 (1), 52499 (2013).
- Chevalier, R., Forbes, M., Thornhill, B. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney International. 75 (11), 1145-1152 (2009).
- Forbes, M., Thornhill, B., Chevalier, R. Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: a new look at an old model. American Journal of Physiology. Renal physiology. 301 (1), 110-117 (2011).
- Yang, H. -. C., Zuo, Y., Fogo, A. B. Models of chronic kidney disease. Drug Discovery Today. Disease Models. 7 (1-2), 13-19 (2010).
- Hackl, M. J., et al. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nature Medicine. 19 (12), 1661-1666 (2013).
- Kitching, A., Kuligowski, M., Hickey, M. In vivo imaging of leukocyte recruitment to glomeruli in mice using intravital microscopy. Methods in Molecular Biology. 466, 109-117 (2009).
- Savin, V. J., Terreros, D. A. Filtration in single isolated mammalian glomeruli. Kidney International. 20 (2), 188-197 (1981).
- Chomczynski, P., Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nature Protocols. 1 (2), 581-585 (2006).
- El Karoui, K., et al. Endoplasmic reticulum stress drives proteinuria-induced kidney lesions via Lipocalin 2. Nature Communications. 7, 10330 (2016).
- VA, M., et al. Multiplexed measurements of gene signatures in different analytes using the Nanostring nCounter Assay System. BMC Research Notes. 2, 80 (2009).
- Springer, A., et al. A combined transcriptome and bioinformatics approach to unilateral ureteral obstructive uropathy in the fetal sheep model. The Journal of Urology. 187 (2), 751-756 (2012).
- Braun, F., Becker, J., Brinkkoetter, P. Live or let die: Is there any cell death in podocytes. Seminars in Nephrology. 36 (3), 208-219 (2016).
- Kim, W. The role of angiopoietin-1 in kidney disease. Electrolyte & Blood Pressure E & BP. 6 (1), 22-26 (2008).
- Liu, F., Zhuang, S. Role of receptor tyrosine kinase signaling in renal fibrosis. International Journal of Molecular Sciences. 17 (5), 972 (2016).
- Martini, S., et al. Integrative biology identifies shared transcriptional networks in CKD. Journal of the American Society of Nephrology: JASN. 25 (11), 2559-2572 (2014).
- Mühlberger, I., et al. Integrative bioinformatics analysis of proteins associated with the cardiorenal syndrome. International Journal of Nephrology. 2011, 809378 (2010).
- Satirapoj, B., et al. Periostin: novel tissue and urinary biomarker of progressive renal injury induces a coordinated mesenchymal phenotype in tubular cells. Nephrology, Dialysis, Transplantation. 27 (7), 2702-2711 (2012).
- Fengxin, Z., et al. Numb contributes to renal fibrosis by promoting tubular epithelial cell cycle arrest at G2/M. Oncotarget. 7 (18), 25604-25619 (2016).
- Sandoval, R. M., Molitoris, B. A. Quantifying glomerular permeability of fluorescent macromolecules using 2-photon microscopy in Munich Wistar rats. Journal of Visualized Experiments: JoVE. (74), e50052 (2013).
- Russo, L. M., et al. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. Journal of the American Society of Nephrology: JASN. 20 (3), 489-494 (2009).
- Russo, L. M., et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney International. 71 (6), 504-513 (2007).
- Sandoval, R. M., Wang, E., Molitoris, B. A. Finding the bottom and using it: Offsets and sensitivity in the detection of low intensity values in vivo with 2-photon microscopy. Intravital. 2 (1), 23674 (2014).
- Kuligowski, M. P., Kitching, A. R., Hickey, M. J. Leukocyte recruitment to the inflamed glomerulus: a critical role for platelet-derived P-selectin in the absence of rolling. Journal of Immunology. 176 (11), 6991-6999 (2006).
- Devi, S., et al. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nature Medicine. 19 (1), 107-112 (2013).
- Finsterbusch, M., et al. Patrolling monocytes promote intravascular neutrophil activation and glomerular injury in the acutely inflamed glomerulus. Proceedings of the National Academy of Sciences of the United States of America. 113 (35), 5172-5181 (2016).
- Shroff, U. N., Gyarmati, G., Izuhara, A., Deepak, S., Peti-Peterdi, J. A new view of macula densa cell protein synthesis. American Journal of Physiology. Renal Physiology. 321 (6), 689-704 (2021).

