Ultrasonic-Assisted Extraction of Cannabidiolic Acid from Cannabis Biomass
Summary
Ultrasonic-assisted extraction (UAE) increases extraction efficiency of solvents and when applied to Cannabis spp. biomass it reduces the time required for extraction. This decreases the cost and potential cannabinoid loss due to degradation. Additionally, UAE is considered a green method due to low solvent use.
Abstract
Industrial hemp (Cannabis spp.) has many compounds of interest with potential medical benefits. Of these compounds, cannabinoids have come to the center of attention, specifically acidic cannabinoids. The focus is turning toward acidic cannabinoids due to their lack of psychotropic activity. Cannabis plants produce acidic cannabinoids with hemp plants producing low levels of psychotropic cannabinoids. As such, utilization of hemp for acidic cannabinoid extraction would eliminate the need for decarboxylation prior to extraction as a source for the cannabinoids. The use of solvent-based extraction is ideal for obtaining acidic cannabinoids as their solubility in solvents such as supercritical CO2 is limited due to the high pressure and temperature required to reach their solubility constants. An alternative method designed to increase solubility is ultrasonic-assisted extraction. In this protocol, the impact of solvent polarity (acetonitrile 0.46, ethanol 0.65, methanol 0.76, and water 1.00) and concentration (20%, 50%, 70%, 90%, and 100%) on ultrasonic-assisted extraction efficiency has been examined. Results show that water was the least effective and acetonitrile was the most effective solvent examined. Ethanol was further examined since it has the lowest toxicity and is generally regarded as safe (GRAS). Surprisingly, 50% ethanol in water is the most effective ethanol concentration for extracting the highest amount of cannabinoids from hemp. The increase in cannabidiolic acid concentration was 28% when compared to 100% ethanol, and 23% when compared to 100% acetonitrile. While it was determined that 50% ethanol is the most effective concentration for our application, the method has also been demonstrated to be effective with alternative solvents. Consequently, the proposed method is deemed effective and rapid for extracting acidic cannabinoids.
Introduction
Industrial hemp (Cannabis spp.) produces acidic cannabinoids in various plant tissues (flowers, leaves, and stems), with the highest concentration found in the flower1. The Cannabis industry utilizes several methods to extract these compounds. One such method is solvent extraction that utilizes a non-polar and/or polar solvent, of which ethanol is the most commonly used. However, solvent extraction alone is limited in its ability; therefore, augmentative extraction techniques, such as microwave-assisted extraction (MAE) and ultrasonic-assisted extraction (UAE), are designed to increase the yield. In addition, high concentration cannabidiol (CBD) can be extracted using supercritical fluid technologies2.
Extraction is a dynamic process, and several factors influence its efficiency, namely moisture content, particle size, and solvent3. Specifically, for the UAE technique, efficiency is governed by temperature, pressure, frequency, and time4.
Ultrasonic-assisted extraction is the process where ultrasonic waves are passed through a liquid to agitate particles. During the agitation process, plant materials experience acoustic cavitation, cycles of compression and expansion which form bubbles that collapse in solution resulting in the generation of extreme temperature and pressure5. The pressure and temperature changes alter the physical properties of the solvents, which can result in increased efficacy of extraction6. Additionally, cavitation can disrupt molecular interactions leading to organic and inorganic compounds leaching from the plant matrix7. The process involves two main types of physical phenomena: (1) diffusion across the cell wall, and (2) rinsing of the cellular contents after breaking the wall8. However, the use of UAE is not without its pitfalls; there are several reports that UAE can degrade compounds9,10. Additionally, the temperatures generated at the cavitation sites are above those necessary for decarboxylation of cannabinoids. However, Mudge et al.11 used UAE and did not observe large decarboxylation of CBD or tetrahydrocannabinol (THC), thereby demonstrating that UAE is an efficient and green method for the extraction of cannabinoids since they can be extracted quickly using low energy.
De Vita et al.12 examined the use of MAE and UAE methods specifically and found that when applying the optimal conditions for each method, UAE extracted more of the acidic and neutral CBD and THC present in the plant material. Similarly, Rožanc et al.13 compared multiple methods of extraction (UAE, soxhlet, maceration, and supercritical fluid) and examined the extracts' biological activity. Rožanc demonstrated that all the methods were effective at extracting cannabinoids; however supercritical fluid and UAE were most effective at extracting cannabidiolic acid (CBDA). Additionally, the UAE extraction had the highest biological activity when measured by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. Rožanc's study also showed that while the extraction processes are effective at producing crude extracts, there remains a portion of non-cannabinoid compounds that influence the extracts' biological activity. Additionally, these compounds can complicate the isolation and purification of individual cannabinoid compounds from the crude extracts13.
Supercritical fluid extraction (SFE) techniques have been used to extract neutral cannabinoids. Several studies demonstrated that SFE plus an organic solvent, such as ethanol, resulted in higher extraction efficiencies of neutral cannabinoids2,3. When the pressure was increased to levels capable of extracting the acidic cannabinoids, non-cannabinoid content also increased. As such, these high pressures are not practical for industrial processing as the selectivity of SFE for cannabinoids decreased and additional post-processing is required. Consequently, decarboxylation must be done prior to SFE, which can result in cannabinoid losses of up to 18%2. To increase efficiencies in SFE, it has been combined with techniques such as solid-phase extraction to increase the purity of the final extract14. However, despite having high purity as the final product, only neutral cannabinoids are obtained.
Traditionally, in the analytical laboratory, cannabinoids were extracted in a 9:1 methanol:chloroform mixture. However, Mudge et al.11 demonstrated that effective extraction can be carried out with single solvents when employing UAE. The study showed that 80% methanol was as effective as the traditional 9:1 methanol:chloroform extraction, thereby indicating that greener solvents can be as effective. As such, UAE was examined for its potential use due to having several benefits, including low capital cost, reduced extraction time, and lower energy use and solvent volumes. However, in the case of UAE, when polar solvents are used, chlorophyll and other non-cannabinoids can be extracted, which may cause a problem in color7. Consequently, to examine the potential for obtaining acidic cannabinoids at a commercial scale, UAE was employed using the industrial hemp variety Cherry Wine. Cherry Wine is a hybrid of C. sativa and C. indica, a cross between the varieties of The Wife and Charlotte's Cherries. The Cherry Wine varietal is a high CBDA producing strain (15% to 25% CBD) with low levels of tetrahydrocannabinolic acid (THCA). The varietal is a C. indica-dominate strain that has 7 to 9 weeks of flowering.
In order to establish the optimal UAE extraction protocol, two approaches were taken: the traditional one factor at a time (OFT) optimization and a Design of Experiment (DoE) approach using a Central Composite Design (CCD)15. For the DoE, CBDA/CBD extraction was optimized based on the sample/solvent ratio, extraction time, and solvent concentration as factors, and the resulting data was analyzed by Response Surface Methodology (RSM). In conclusion, the protocol described outlines the optimal method for extracting the highest amount of CBDA/CBD.
Protocol
1. Plant material preparation
- Obtain Cherry Wine inflorescences from plants grown in the field, planted in a south-to-north configuration, with plants 1 m apart in center and rows 1.2 m apart (cultivation located in Longmont, Colorado, USA).
- Air-dry the inflorescences at 35 °C for 48 h. Grind the inflorescences using a grinding machine set a 177 µm.
- Pass the pulverized material through the No. 80 mesh sieve. Store the resulting powder in a sealed bag at room temperature for future use.
2. Ultrasound extraction
- Weigh 0.5 g of the Cannabis inflorescence powder into a 50 mL conical tube. Add 40 mL of the solvent (e.g., 50% ethanol in deionized water) to the vessel.
- Place the extraction vessel in the ultrasonic bath set at 40 kHz and at room temperature (sonication power is 100 W).
- Perform the extraction in the ultrasonic bath for 30 min, increasing the temperature of the bath from 25 °C to 30 °C.
- Decant the extraction fluid into a centrifuge tube.
- Centrifuge the fluid at 3,000 x g at 15 °C for 15 min. Filter the supernatant under vacuum through an 8 µm filter paper.
3. High-performance liquid chromatography (HPLC) quantitative analysis
- Dilute seven cannabinoid standards: cannabichromene (CBC), CBD, CBDA, cannabinol (CBN), tetrahydrocannabinolic acid (THCA), Δ8-THC, and Δ9-THC to operating concentrations of 100, 50, 25, and 12.5 µg/mL in 100% methanol. Mix and sonicate for 5 min in an ultrasonic bath set at 40 kHz and sonication power of 100 W
- Filter the standards through a 0.45 µm polytetrafluoroethylene (PTFE) syringe filter. Filter the sample supernatant (from step 2.5) through a 0.45 µm PTFE syringe filter.
- Put the sample to be analyzed in a 1.5 mL vial into the HPLC autosampler and load 10 µL at a time.
- Run HPLC as per the conditions and parameters provided in Table 1. Derive cannabinoid concentrations in 50-200 µg/mL from the generated standard curve.
- Multiply with the volume of the solvent (40 mL) used in the extraction process to obtain µg of cannabinoid. Convert the µg of cannabinoid to mg of cannabinoid by dividing it by 1000.
- Divide with the original weight of the plant material (0.5 g) used in the extraction to obtain mg/g dry weight.
4. Optimization using response surface methodology
- Establish the model, consisting of 15 experimental runs with 12 factorial points and three center points as shown in Table 4 using a data analysis tool.
- Optimize the extraction parameters, extraction time (T), solvent concentration (S), and sample/solvent ratio (R) using response surface methodology and central composite design. Set the range of variables T, S, and R as 5-30 min, 20%-100%, and 60-100 (1:X), respectively.
- Select the total yield of lipophilic extract and the yields of extracted CBD and CBDA as response factors (RF).
Representative Results
The solvents utilized range from the middle of the polarity index (0.460 – ACN) to polar (1.000 – water). From Table 2, it can be seen that water did not make an effective extractant for cannabinoids, which is not unexpected, as cannabinoids have limited solubility in water due to their hydrophobicity13. In contrast to water, the other solvents had similar extracted values of CBD and CBDA, with the least polar solvent acetonitrile (ACN) having higher extraction when compared to the two alcohols. While statistically lower, ethanol was able to extract nearly 93% and 99% increase in the major cannabinoid CBDA extracted by ACN and methanol, respectively. Furthermore, the ethanol extract had the lowest levels of THCA, the precursor of Δ9-THC. Decreased levels of THCA were desired in order to limit the potential for conversion to the psychotropic Δ9-THC, a concern for industrial applications16. While all the organic solvents are generally regarded as safe (GRAS), only ethanol has no limitation to its quantity in the final product2. The difference between the values obtained for ethanol and methanol would warrant that either of them could be used, however, the lower toxicity of ethanol makes it a better choice for commercial use. Likewise, while ACN yielded more cannabinoids in the extract, the low levels of residual ACN permitted did not justify its use in the light of the additional purification to remove trace amounts when there was only a 7% gain in CBDA concentration.
Examination of the impact that an aqueous ethanol solution has on cannabinoid concentration is shown in Table 3. It has been shown that the solution concentration can influence the extraction efficiency17,18. The extraction of cannabinoids using UAE is no exception. Maximum extraction was observed at the 50% ethanol concentration. This represents a 39.7% increase over 100% ethanol for the extraction of CBDA. Additionally, 50% ethanol also reduced the levels of THCA extracted by 20.3%.
To confirm the results from OFT optimization, DoE (Table 4) using RSM was examined as shown in Table 5. RSM analysis (Figure 1) confirmed a 30 min extraction and a 1:100 sample to solvent ratio. The RSM analysis resulted in an ideal concentration of 53.4% ethanol in water. This confirms the 50% obtained by the OFT. While the optimum ethanol concentration by DoE was found to be slightly higher than the 50% by OFT, 50% ethanol was used in the protocol due to its convenience of preparation and the negligible decrease in the overall CBDA/CBD extraction.
Results obtained using 50% ethanol for UAE were compared to results obtained using 50% ethanol for maceration (weigh 0.5 g of the Cannabis inflorescence powder into a 50 mL tube and add 50 mL of 50% ethanol to the vessel) alone as shown in Table 6. The resulting CBDA extraction amount was about 55% higher in the UAE sample compared to the maceration sample. Additionally, it is important to note that there was a doubling of the CBD concentration extracted as well.
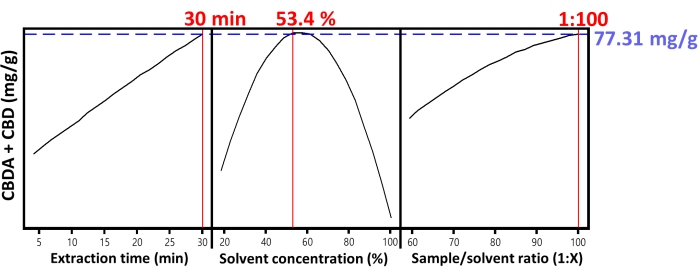
Figure 1. Optimization graph. Optimization graph of a response surface for extraction time, solvent concentration, and sample/solvent ratio of Cannabis. The black line indicates the y-value plotted, the blue line indicates the maximum y-value, and its numerical value is also mentioned in blue, and the red line indicates the x-value when the y-value is the maximum, and the numerical values for all these parameters is mentioned in red at the top of each graph. Please click here to view a larger version of this figure.
Table 1. Parameters utilized for HPLC cannabinoid analysis. Please click here to download this Table.
Table 2. Individual cannabinoids of extracts derived from 100% solvents analyzed by HPLC (mg/g dry weight). Please click here to download this Table.
Table 3. Individual cannabinoids of extracts derived from aqueous ethanol were analyzed by HPLC (mg/g dry weight). Please click here to download this Table.
Table 4. Experimental data on CBDA + CBD of Cannabis based on central composite design by response surface method. Please click here to download this Table.
Table 5. Polynomial equations calculated by RSM program for extraction conditions of Cannabis. Please click here to download this Table.
Table 6. Ultrasonic-assisted extraction compared with maceration extraction (without ultrasonic) of individual cannabinoid quantity (mg/g) in extracts from 50% ethanol solvent. Please click here to download this Table.
Discussion
The polarity of a solvent plays a critical role in the effective extraction of compounds. Since acidic cannabinoids are slightly polar in nature, due in large part to the carboxylic acid moiety, it was assumed that a polar solvent such as methanol or ethanol would be most effective. Garrett and Hunt19, in their study using THC, demonstrated that solubility in aqueous ethanol was based on percent ethanol in the solution and ionic strength of the solution. While ionic strength was not examined in the current study, it can be assumed that it played an important role in the increased extraction efficiency at 50% ethanol. Additionally, as demonstrated by Garrett and Hunt19, pH has an impact on the solubility in aqueous solutions. Metcalf20 also emphasizes the importance of pH where they showed that the pKa of cannabidiol in an aqueous solution was between 8.0 and 8.5 in contrast to other reports of the pKa being from 9.13 to 9.64.
Further supporting the use of aqueous solutions is the practice of solventless extraction using water. The process involves the dynamic maceration of the Cannabis to dislodge the trichomes from the plant material21. The trichomes and extractant can then be dried to result in a hash product available for further processing. In the current study, the use of UAE provides the means for the release of the trichomes contents. Utilizing an aqueous solution instead of water allows for better solubilization of the acidic cannabinoids. An additional benefit associated with UAE is its ability to extract and retain the acidic cannabinoids in their original form22. Lewis-Bakker et al.22 also demonstrated that UAE was more efficient at extracting CBDA than SFE or soxhlet.
Brighenti et al.23 found in non-decarboxylated hemp that there was no significant difference in individual cannabinoids extracted by several techniques with room temperature ethanol performing slightly better as an extraction solvent. Consequently, the Brighenti23 study and the current study both employed ethanol as the solvent of choice. The choice of ethanol in this study was further supported by the anticipated downstream processing methods to be employed. Ethanol’s selection is compatible with the winterization process to be employed and allows for the concentration of the extract and purification using methods such as flash or centrifugal partition chromatography3. Additionally, any trace amounts of ethanol are not of concern due to the acceptable limits associated with its use24.
Solvent concentration influences the extraction process and was determined to be the most important factor in the protocol. Dilution of an organic solvent with water produces a solvent with a modified polarity and sometimes modified physicochemical properties. Water with a polarity of 1.00 has unique features in that as temperature increases, the dielectric constant decreases and so does the polarity5. Additionally, an increase in temperature reduces the surface tension and viscosity, thereby improving penetration of the matrix17. Lastly, an increase in water temperature improves the analyte diffusion and mass-transfer kinetics of an extraction17. The main force in UAE is ultrasonic waves that generate heat via compression and release from sound pressure changes. The high temperatures experienced within the bubbles are mitigated by the presence of alcohol as seen by Rae25. The presence of alcohol in the bubble increases the heat capacity of the gaseous mixture25. Consequently, this improves the extractability of water, and also causes cavitation of micro-bubbles, thereby disrupting cellular walls allowing for easier solvent extraction.
The literature contains multiple methods for the extraction of cannabinoids4,17,26,27,28. Conventional methods, such as maceration in ethanol (with no ultrasonication), are still employed widely due to their ease and the costs associated with modern methods, such as supercritical fluid21. Ultrasound-assisted extraction provides the opportunity to enhance conventional solvent extraction methods with a modern extraction technique designed to enhance yield. Ultrasound-assisted extraction allows for the use of green solvents (i.e., water, ethanol, etc.), enhanced yields, and reduced time and costs. The use of UAE as a pretreatment to other extraction techniques is still widely unexplored. However, a 24% increase in a crude extract yield was obtained using UAE before soxhlet extraction28, thereby demonstrating the potential for combined methods of extraction. The currently proposed method focuses on the extraction of acidic cannabinoids from industrial hemp utilizing UAE alone, however, the potential for further utilization in combination with other alternative and conventional extraction methods provides interesting paths for future research.
Conclusively, from this study, it was established how various extraction solvents and extraction solvent ratios affect cannabinoid extraction. UAE methodology was employed to examine select solvents, based on permissible quantities in the final product, for potential application in the industry. Based on these findings, UAE employment resulted in higher extraction of cannabinoids compared to maceration. Additionally, it was observed using DoE and RSM that 53.4% ethanol was found to have higher extraction of cannabinoids compared to other ethanol concentrations. Consequently, these findings suggest that UAE is efficacious as a means to increase cannabinoid extraction and therefore should be examined further at industrial capacity.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Institute of Cannabis Research at Colorado State University-Pueblo, the Korea Innovation Foundation grant funded by the Korean government (MSIT) (2021-DD-UP-0379), and Chuncheon city (Hemp R&D and industrialization, 2020-2021).
Materials
| Acetonitrile | J.K.Baker | 9017-88 | solvent |
| Cannabichromene | Cerilliant | C-143 | Cannabinoids standard |
| Cannabidiol | Cerilliant | C-045 | Cannabinoids standard |
| Cannabidiolic acid | Cerilliant | C-144 | Cannabinoids standard |
| Cannabidivarin | Cerilliant | C-140 | Cannabinoids standard |
| Cannabigerol | Cerilliant | C-141 | Cannabinoids standard |
| Cannabinol | Cerilliant | C-046 | Cannabinoids standard |
| Centrifuge | Hanil Scientific Inc | Supra 22K | Centrifuge |
| Cherry Wine hemp | CFH, Ltd. | – | Flower extraction material |
| Distilled water | TEDIA | WS2211-001 | solvent |
| Ethanol | TEDIA | ES1431-001 | solvent |
| Filter paper | Whatman | #2 | Filtering |
| Grinder | Daesung Artlon | DA280-S | Milling |
| HPLC | Shimadzu | LC-10 system | Analysis of Cannabinoid |
| Methanol | TEDIA | MS1922-001 | solvent |
| Minitab 16.2.0 | Minitab Inc. | ||
| Syringe filters | Whatman | 6779-1304 | Filtering |
| Tetrahydrocannabivarin | Cerilliant | T-094 | Cannabinoids standard |
| Trifluoroacetic acid | Sigma-aldrich | 302031-1L | HPLC flow solvent |
| Untrasonic bath | Jinwoo | 4020P | Ultrasonic extraction |
| Zorbax Eclipse plus C18 HPLC column | Agilent | 9599990-902 | HPLC column |
| Δ8 – Tetrahydrocannabinol | Cerilliant | T-032 | Cannabinoids standard |
| Δ9 – Tetrahydrocannabinol | Cerilliant | T-005 | Cannabinoids standard |
| Δ9 – Tetrahydrocannabinolic acid | Cerilliant | T-093 | Cannabinoids standard |
Referenzen
- Hemphill, J. K., Turner, J. C., Mahlberg, P. G. Cannabinoid content of individual plant organs from different geographical strains of Cannabis sativa L. Journal of Natural Products. 43 (1), 112-122 (1980).
- Baldino, L., Scognamiglio, M., Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. Journal of Supercritical Fluids. 165, 104960 (2020).
- Daniel, R. G., et al. Supercritical extraction strategies using CO2 and ethanol to obtain cannabinoid compounds from cannabis hybrid flowers. Journal of CO2 Utilization. 30, 241-248 (2019).
- Azmir, J., et al. Techniques for extraction of bioactive compounds from plant materials: A review. Journal of Food Engineering. 117 (4), 426-436 (2013).
- Ohl, C. D., Kurz, T., Geisler, R., Lindau, O., Lauterborn, W. Bubble dynamics, shock waves and sonoluminescence. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 357 (1751), 269-294 (1999).
- Castro-Puyana, M., Marina, M. L., Plaza, M. Water as green extraction solvent: Principles and reasons for its use. Current Opinion in Green and Sustainable Chemistry. 5, 31-36 (2017).
- Herrera, M. C., De Castro, M. L. Ultrasound-assisted extraction of phenolic compounds from strawberries prior to liquid chromatographic separation and photodiode array ultraviolet detection. Journal of Chromatography A. 1100 (1), 1-7 (2005).
- Mason, T. J., Paniwnyk, L., Lorimer, J. P. The uses of ultrasound in food technology. Ultrasonics Sonochemistry. 3 (3), 253-260 (1996).
- Soares, V. P., et al. Ultrasound assisted maceration for improving the aromatization of extra-virgin olive oil with rosemary and basil. Food Research International. 135, 109305 (2020).
- Kshitiz, K., et al. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrasinics Sonochemistry. 70, 105325 (2017).
- Mudge, E. M., Murch, S. J., Brown, P. N. Leaner and greener analysis of cannabinoids. Analytical and Bioanalytical Chemistry. 409 (12), 3153-3163 (2017).
- De Vita, D., et al. Comparison of different methods for the extraction of cannabinoids from cannabis. Natural Product Research. 34 (20), 2952-2958 (2020).
- Rožanc, J., et al. Different Cannabis sativa extraction methods result in different biological activities against a colon cancer cell line and healthy colon cells. Plants. 10 (3), 566 (2021).
- Karğili, U., Aytaç, E. Supercritical fluid extraction of cannabinoids (THC and CBD) from four different strains of cannabis grown in different regions. The Journal of Supercritical Fluids. 179, 105410 (2022).
- Sushma, C., et al. Optimization of ultrasound-assisted extraction (UAE) process for the recovery of bioactive compounds from bitter gourd using response surface methodology (RSM). Food and Bioproducts Processing. 120, 120-122 (2022).
- David, J. P., et al. Potency of Δ9-THC and Other Cannabinoids in Cannabis in England in 2005: Implications for Psychoactivity and Pharmacology. Journal of Forensic Sciences. 11, 129 (2008).
- Agarwal, C., Máthé, K., Hofmann, T., Csóka, L. Ultrasound-assisted extraction of cannabinoids from Cannabis Sativa L. optimized by response surface methodology. Journal of Food Science. 83 (3), 700-710 (2018).
- Oroian, M., Ursachi, F., Dranca, F. Influence of ultrasonic amplitude, temperature, time and solvent concentration on bioactive compounds extraction from propolis. Ultrasonics Sonochemistry. 64 (2020), 105021 (2020).
- Garrett, E. R., Hunt, A. Physiochemical properties, solubility, and protein binding of Δ9-tetrahydrocannabinol. Journal of Pharmaceutical Sciences. 63 (7), 1056-1064 (1974).
- Metcalf, D. G. Chemical Abstracts. United States patent. , (2020).
- Lazarjani, M. P., Young, O., Kebede, L., et al. Processing and extraction methods of medicinal cannabis: a narrative review. Journal of Cannabis Research. 3 (1), 1-15 (2021).
- Lewis-Bakker, M. M., Yang, Y., Vyawahare, R., Kotra, L. P. Extractions of medical cannabis cultivars and the role of decarboxylation in optimal receptor responses. Cannabis and Cannabinoid Research. 4 (3), 183-194 (2019).
- Brighenti, V., Pellati, F., Steinbach, M., Maran, D., Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L.(hemp). Journal of Pharmaceutical and Biomedical Analysis. 143, 228-236 (2017).
- . FDA Available from: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=SCOGS (2021)
- Rae, J., et al. Estimation of ultrasound induced cavitation bubble temperatures in aqueous solutions. Ultrasonics Sonochemistry. 12, 325-329 (2005).
- Moreno, T., Montanes, F., Tallon, S. J., Fenton, T., King, J. W. Extraction of cannabinoids from hemp (Cannabis sativa L.) using high pressure solvents: An overview of different processing options. Journal of Supercritical Fluids. 161, 104850 (2020).
- Zhang, Q. W., Lin, L. G., Ye, W. C. Techniques for extraction and isolation of natural products: a comprehensive review. Chinese Medicine. 13 (20), 1-26 (2018).
- Fathordoobady, F., Singh, A., Kitts, D. D., Singh, A. P. Hemp (Cannabis sativa L.) extract: Anti-microbial properties, methods of extraction, and potential oral delivery. Food Reviews International. 35 (7), 664-684 (2019).

