The Establishment and Utilization of Patient Derived Xenograft Models of Central Nervous System Metastasis
Summary
Central nervous system metastasis PDX models represent the phenotypic and molecular characteristics of human metastasis, making them excellent models for preclinical studies. Described here is how to establish PDX models and the inoculation routes that are best used for preclinical studies.
Abstract
The development of novel therapies for central nervous system (CNS) metastasis has been hindered by the lack of preclinical models that accurately represent the disease. Patient derived xenograft (PDX) models of CNS metastasis have been shown to better represent the phenotypic and molecular characteristics of the human disease, as well as better reflect the heterogeneity and clonal dynamics of human patient tumors compared to historic cell line models. There are multiple sites that can be used to implant patient-derived tissue when setting up preclinical trials, each with their own advantages and disadvantages, and each suited for studying different aspects of the metastatic cascade. Here, the protocol describes how to establish PDX models and present three different approaches for utilizing CNS metastasis PDX models in pre-clinical studies, discussing each of their applications and limitations. These include flank implantation, orthotopic injection in the brain, and intracardiac injection. Subcutaneous flank implantation is the easiest to monitor and, therefore, most convenient for pre-clinical studies. In addition, metastases to brain and other tissues from flank implantation were observed, indicating that the tumor has undergone multiple steps of metastasis, including intravasation, extravasation, and colonization. Orthotopic injection in the brain is the best option for recapitulating the brain tumor microenvironment and is useful for determining the efficacy of biologics to cross the blood-brain barrier (BBB) but bypasses most steps of the metastatic cascade. Intracardiac injection facilitates metastasis to the brain and is also useful for studying organ tropism. While this method forgoes earlier steps of the metastatic cascade, these cells will still have to survive circulation, extravasate, and colonize. The utility of a PDX model, is therefore, impacted by the route of tumor inoculation and the choice of which one to utilize should be dictated by the scientific question and overall goals of the experiment.
Introduction
The incidence of metastasis to the central nervous system (CNS) has increased over recent years1,2,3. Traditional therapies for CNS metastasis, such as tumor resection, whole brain radiotherapy, and stereotactic radiosurgery, have been largely palliative and rarely curative, and can lead to debilitating side effects, such as cognitive deterioration1. Recently, many new targeted and immunological therapies are being developed for the treatment of CNS metastasis that show promise in being more effective treatments, while having fewer side effects4.
Translating preclinical results into meaningful clinical endpoints often requires effective and predictive modeling strategies. Historically, cell line xenograft models were the standard for preclinical research in CNS metastasis research. However, these cell line models do not reflect the true tumor behavior of the host tumor or represent the histological or molecular heterogeneity of the disease. Furthermore, cell line models are able to adapt to in vitro growth conditions and, therefore, lose the original properties of the host tumor. Patient derived xenografts (PDXs), which engraft a patient's tumor into an immunodeficient or humanized mouse, are increasingly used in translational cancer research. Researchers have shown that PDX models can usually faithfully recapitulate tumor growth, histological characteristics, maintain tumor heterogeneity, metastatic potential, and molecular genetic features. In addition, PDX models are prognostic whereby the PDX tumor latency period correlates with patient overall survival and they have also been shown to accurately predict therapeutic response in patient trials5,6.
There has been an emergence of CNS metastasis PDXs. Mostly, these have been developed representing tumors originating from a single origin, such as non-small cell lung cancer (NSCLC)7, breast cancer8,9, and melanoma10,11. More recently, a large and diverse collection of PDX models have been developed and characterized, representing eight different histological subtypes12. It has been demonstrated that PDX models for CNS metastasis closely resemble their original patient tumor, both histologically and molecularly and have also demonstrated histological unique differences and similarities10,12. Furthermore, while most CNS metastasis PDX models maintain the clonal heterogeneity of human tumors, some displayed evidence of clonal succession12, making them also ideal for studying resistance to therapies by monitoring clonal changes after treatment.
The protocols described here outline methods of PDX establishment and various routes of inoculation used in preclinical studies of CNS metastasis (Figure 1). These implantation methods vary in their ability to mimic growth and metastasis. Here, the protocol highlights the applications for each route of implantation and demonstrates how they could be used for the study of CNS metastasis.
Protocol
The following are a series of step-by-step protocols used for both establishing PDX models by subcutaneous flank implantation and for setting up preclinical studies that enable testing of treatments, which can aid in assessing biological changes and various steps of the metastatic cascade. All the studies and models used 3-8 weeks old female NOG mice. All the tissue samples were collected under informed consent in accordance with a protocol approved by the Institutional Review Board (IRB). All animal experiments were performed in accordance with an Institutional Animal Care and Use Committee (IACUC) approved protocol.
1. Establishment and propagation of PDX models by flank implantation
- Establishment of PDX models
- Following surgical resection of the tumor from the patient in the operating room, store the fresh tumor tissues in a suitable solution (such as DMEM) and immediately place it on ice. Use an excess of storage solution (>10 mL) to ensure that the tissue is fully submerged.
- Transfer tissue to tissue culture dish and rinse with 5 mL DPBS.
NOTE: This step should be conducted in a biosafety cabinet using aseptic techniques. Precautions should be taken by wearing appropriate personal protective equipment (PPE) to protect against possible human infectious agents. - Remove necrotic regions from the tumor.
NOTE: This can be recognized as a white mushy region toward the center of the tissue. - Cut the remaining tissue into approximately 2 x 2 x 2 mm pieces.
- Transfer the tissues into a microcentrifuge tube containing the growth factor reduced basement membrane matrix and store them on ice. Ensure that sufficient basement membrane matrix (>200 µL) is used to fully submerge each tissue piece.
- Cryopreserve the remaining tissues that will not be implanted according to protocol described in the step 1.3.
- Anesthetize the animal in an induction chamber with 2-5% isoflurane and oxygen. Once anesthetized, transfer the animal to a nose cone to maintain anesthesia at 1.5-2.5% isoflurane with a continuous oxygen supply. Confirm the anesthesia depth via lack of pedal reflex. Apply the veterinary ophthalmic ointment to prevent eye dryness during surgery. Provide thermal support for the animal throughout the procedure until the animal recovers.
- Identify the implantation site on the mouse.
NOTE: This should be on the right or left flank of the mouse, generally landmarked laterally on the side of the abdominal region, caudal to the ribcage. - To prepare the surgical area, shave the fur and disinfect with three alternating scrubs of povidone iodine and 70% ethanol.
- Using forceps, lift the skin of the mouse and make a 0.5-1 cm incision on the skin.
- Insert a pair of surgical scissors slowly under the skin at the incision site to create a pocket (0.5-1 cm deep) in the subcutaneous space.
- Carefully place one tumor piece in the pocket and push it push it to the bottom of the pocket to prevent the tumor from slipping out.
- Close the incision using 4-0 nylon surgical sutures.
NOTE: Other wound closure methods such as non-absorbable or absorbable sutures and wound clips can also be used. - Transfer the mouse back to the cage and monitor the animal's recovery from anesthesia, until it is ambulatory.
NOTE: Analgesics are not required but can be administered if pain is observed in the mice. - Monitor the tumor growth weekly. One tumor is expected per mouse.
NOTE: The tumor's volume at transplantation will seem to initially decrease but this is not a cause for concern. A tumor is considered to have taken once it becomes palpable and enters a logarithmic growth phase. This first passage represents the F0 generation. - Once the tumors start growing, measure the tumors three times per week. Measure the length and width of the tumors with a caliper. To calculate the volume of the tumor, use the formula: length x width x width / 2.
- Euthanize the mice implanted with PDX tumors when the tumors are greater than 15 mm in diameter using the longest side of the tumor. Perform the euthanasia by CO2 inhalation in a CO2 induction chamber, followed by cervical dislocation as a secondary method.
- Resect the tumor from the flank of the animal by making an incision. Dissect the excised tumor gently with blunt scissors and forceps. To do this, first cut off the skin on top of the tumor, then cut the tumor away from the muscle layer below it.
- Transfer the tumor tissue to >10 mL of a suitable storage solution (such as DMEM). Immediately place it on ice. This tumor can be cryopreserved or passaged to another set of mice. Consider this passage as F1.
NOTE: Passaging again would render the tumor passage F2 and so on.
- Propagation of PDX models
- Begin with the resected tumor kept in storage solution from step 1.1.19.
- Transfer the tissue to a tissue culture dish and rinse with 5 mL DPBS.
- Remove the necrotic regions from the tumor.
- Cut the tissue into approximately 2 x 2 x 2 mm pieces.
- Transfer the tissues into a microcentrifuge tube containing growth factor reduced basement membrane matrix (>200 µL) and store on ice.
- Cryopreserve the remaining tissue that is not used for propagation according to the protocol described in step 1.3.
- Anesthetize the animal in an induction chamber with 2-5% isoflurane and oxygen. Once anesthetized, transfer the animal to a nose cone to maintain anesthesia at 1.5-2.5% isoflurane with a continuous oxygen supply. Confirm the anesthesia depth via lack of pedal reflex. Apply the veterinary ophthalmic ointment to prevent eye dryness during surgery. Provide thermal support for the animal throughout the procedure until the animal recovers.
- Identify the implantation site on the mouse.
NOTE: This should be on the right or left flank of the mouse, generally in the abdominal region, caudal to the ribcage. - To prepare the surgical area, shave the fur and disinfect with three alternating scrubs of povidone iodine and 70% ethanol.
- Make a 0.5-1 cm incision on one flank of the mouse.
- Insert a pair of surgical scissors slowly under the skin at the incision to create a pocket (0.5-1 cm deep) in the subcutaneous space.
- Carefully place one tumor piece in the pocket and push it to the bottom of the pocket to prevent the tumor from slipping out.
- Close the incision using 4-0 nylon surgical sutures or other wound closure methods.
- Transfer the mouse back to the cage and monitor its recovery from anesthesia, until it is ambulatory.
NOTE: Analgesics are not required but can be administered if pain is observed in the mice. - During latency (non-growth phase), monitor the tumor growth weekly. One tumor is expected per mouse.
NOTE: The tumor's volume at transplantation will seem to initially decrease but this is not a cause for concern. A tumor is considered to be taken once it becomes palpable and starts to continually grow. - Once the tumors start growing, measure the tumors three times per week. Measure the length and width of the tumors with a caliper. Calculate the volume of the tumor using the formula: length x width x width / 2.
- Euthanize the mice implanted with PDX tumors when the tumors are greater than 15 mm in diameter using the longest side of the tumor. Perform euthanasia by CO2 inhalation in a CO2 induction chamber, followed by cervical dislocation as a secondary method.
- Resect the tumor from the flank of the animal by making an incision and dissecting the tumor out gently with blunt scissors and forceps.
- Transfer the tumor tissue to >10 mL of a suitable storage solution (such as DMEM) and then immediately place on ice.
- Cryopreservation of PDX tumors
- Euthanize the mice implanted with PDX tumors when the tumors are greater than 15 mm in diameter using the longest side of the tumor. Perform euthanasia by CO2 inhalation in a CO2 induction chamber, followed by cervical dislocation as a secondary method.
- Resect the tumor from the flank of the animal by making an incision and dissecting the tumor out gently with blunt scissors and forceps.
- Transfer the tumor tissue to >10 mL of a suitable storage solution (such as DMEM) and then immediately place on ice.
- Transfer the tissue to a tissue culture dish and rinse with 5 mL DPBS.
- Remove necrotic regions from the tumor.
- Cut the tissue into approximately 2 x 2 x 2 mm pieces.
- Transfer the tissue into cryotubes containing 20% DMEM, 70% FBS, and 10% DMSO.
- Transfer the cryotubes to a cryopreservation container and place in a -80 °C freezer.
- When the cryotubes are chilled to -80 °C, transfer them to liquid nitrogen storage.
2. Inoculation routes for preclinical studies
- Subcutaneous flank implantation.
NOTE: Subcutaneous flank implantation can be used for ease and can be helpful for studying all steps of the metastatic cascade.- Use growing PDX tumors or cryopreserved PDX tumors for initial flank implantation.
- For growing PDX tumors, euthanize the mice using an IACUC-approved method when the tumors are greater than 15 mm in length; resect the tumor and transfer the tumor tissue to a suitable storage solution (such as DMEM) and immediately place on ice.
- For cryopreserved PDX tumors, thaw the cryopreserved PDX tissue quickly by immersing in 37 °C water bath.
- Follow the steps 1.2.2-1.2.19.
- Orthotopic implantation by intracranial injection into the brain.
NOTE: This model can be used for testing efficacy of drugs to cross the BBB and to study tumor colonization. This section primarily references the use of the tumor dissociation kit (see Table of Materials). Different tissue types require different dissociation protocols. It is recommended that the user test and optimize the protocol to maximize the dissociation efficiency.- Euthanize the mice implanted with PDX tumors using an IACUC-approved method when the tumors are greater than 15 mm in length.
- Under sterile conditions in a biosafety cabinet, surgically resect the PDX tumors and store in DMEM on ice.
- Prepare the dissociation solution in the appropriate tube by adding the enzyme mix into DMEM as indicated by the manufacturer's protocol.
- Wash the tumor in 5 mL DPBS in a tissue culture dish.
- Remove the necrotic regions from the tumor.
- Cut the tumor into small pieces of 2-4 mm in length.
- Transfer the tumor pieces into the tube containing the enzyme mix.
- Attach the tube to the tissue dissociator and run the program suitable for the tissue type. Consult the manufacturer's protocol for the appropriate program to run, and the dissociation time required.
- After completion of the program, strain the cells through a 70 µm cell strainer.
- Wash the cell strainer with 20 mL of DMEM.
- Centrifuge the dissociated cells at 300 x g for 7 min.
- Aspirate the supernatant and resuspend the cells in DPBS.
- Count the cells and dilute to the required concentration.
- Set up the stereotaxic frame according to the manufacturer's instructions and prepare a heating pad for the mice. Sanitize all areas with 70% ethanol.
- Anesthetize the animal in an induction chamber with 2-5% isoflurane and oxygen. Once anesthetized, transfer the animal to a nose cone to maintain anesthesia at 1.5-2.5% isoflurane with a continuous oxygen supply. Confirm the anesthesia depth via lack of pedal reflex. Apply the veterinary ophthalmic ointment to prevent eye dryness during surgery. Provide thermal support for the animal throughout the procedure until the animal recovers.
- Prepare the surgical area by shaving the fur on the mouse's head to expose the scalp.
- Provide the mouse with the appropriate analgesic, such as one dose of 1 mg/kg Buprenorphine sustained release (SR) administered by subcutaneous injection.
- Transfer the mouse to the stereotaxic frame. Ensure that the mouse is biting on the bite-block. Use the ear bars to firmly secure the head of the mouse.
NOTE: The mouse is properly secured if the head does not move when pushed gently with forceps. - Disinfect the shaved area with three alternating scrubs of povidone-iodine and 70% ethanol.
- Make a 5-7 mm longitudinal incision on the scalp to expose the skull and retract the scalp.
- Scrape off the periosteum with a blunt surgical instrument such as forceps.
- Locate the bregma on the skull.
- Position the needle of the stereotactic frame on top of the bregma and reset the coordinates to 0 or note the coordinate on the arm.
- Move the arm 1 mm posterior (caudally) and 1 mm lateral, right of the midline.
- Mark this location with a permanent marker. If the arm contains a slot for the syringe, attach a marker to the syringe slot to mark the location.
- Drill a small burr hole in the skull at this location. Do not apply too much pressure to prevent drilling into the brain.
- Load a 5 µL, 26 G Hamilton syringe with 5-10 x 104 cells, in a volume of 1-2 µL, and attach to the stereotaxic arm.
- Slowly insert the Hamilton syringe needle 2 mm into brain.
- Begin injecting cells at the desired rate, usually 0.2-0.5 µL/min.
- After the injection is complete, slowly retract the needle from the brain.
- Fill the burr hole with bone wax.
- Close the incision with surgical sutures or surgical glue.
- Transfer the mouse back to the cage and monitor its recovery from anesthesia.
- Monitor the condition of the animals regularly and euthanize them when the humane endpoint criteria are reached in the approved protocol.
NOTE: Successful tumor growth in the brain results in deteriorating condition of the animal, which often manifests with head tilt, rough hair coat, hunched body, squinted eyes, reduced activity, and low body condition score (BCS < 2). - Perform a necropsy on the euthanized animals, followed by histological analysis to confirm the presence of tumors in the brain. Record the length of time it takes from implantation to euthanasia.
- Implantation of PDX models by intracardiac injection
NOTE: This model can be used to study organ tropism once tumor cells are in circulation. This section also uses the Tumor Dissociation Kit and requires optimization by tissue type.- Follow the steps 2.2.1-2.2.13.
- Anesthetize the animal in an induction chamber with 2-5% isoflurane and oxygen. Once anesthetized, transfer the animal to a nose cone to maintain anesthesia at 1.5-2.5% isoflurane with a continuous oxygen supply. Confirm the anesthesia depth via lack of pedal reflex. Apply the veterinary ophthalmic ointment to prevent eye dryness during surgery. Provide thermal support for the animal throughout the procedure until the animal recovers.
- Then, position the mouse in a supine position.
- To prepare the surgical area on the chest, shave the fur and disinfect it with three alternating scrubs of povidone-iodine and 70% ethanol.
- Draw 0.5-10 x 105 cells into a syringe on a 28 G needle, up to a volume of 100 µL.
NOTE: The number of cells required varies depending on the aggressiveness of the model and the optimal number of cells should be empirically determined for each model. - Locate the injection site (slightly left of the mouse's sternum and halfway between the sternal notch and xyphoid process).
- Insert the needle vertically into the mouse at the injection site.
- Observe the successful entry into the left ventricle through a backflow of blood entering the syringe. Slowly dispense the cells into the left ventricle without moving the needle.
- Slowly pull the needle out vertically from the mouse.
- Apply a piece of sterile gauze over the injection site and apply pressure for around 1 min until the bleeding stops, while still allowing chest movement for respiration.
- Remove the mouse from anesthesia and allow it to recover on a heated pad.
NOTE: Successful tumor growth results in deteriorating condition of the animal, which often manifests with ruffled hair coat, hunched body, squinted eyes, reduced activity, and low body condition score (BCS < 2). - Monitor the condition of the animals regularly and euthanize them when the humane endpoint criteria are reached in the approved protocol.
- Perform a necropsy to identify metastases on the euthanized animals, followed by histological analysis to confirm the presence of tumors in the target organ. Record the length of time it takes from intracardiac injection to euthanasia.
Representative Results
Flank-propagated PDX tumors are the easiest to implant, monitor, and resect, and is generally recommended for initial establishment and propagation of PDX tumors (Figure 1). When establishing or propagating PDX tumors, it is prudent to implant tumors in multiple animals, as the tumor take rate may vary and not every piece of tumor will always take in mice. Methods have been developed for the establishment and propagation of CNS metastasis PDXs directly in the brain13. However, these methods are still more challenging with lower take rates and the tumors are significantly more difficult to propagate and monitor than flank implantation.
If patient tumors are not readily available, CNS metastasis PDX tumors can also be obtained from a variety of sources, including repositories of academic labs or commercial companies. After acquiring the tumors, the first priority would be to propagate and cryopreserve as much material as possible, ensuring a great number of low passage tumors are preserved. This ensures that sufficient material is available for an indefinite number of subsequent studies with the PDX models. Much like immortalized cell lines, PDX tumors should be cryopreserved and used at low passage numbers, as genetic drifting results in changes to the phenotype and genotype of the PDXs over time12,14,15. Regardless of the source of PDX tumors, it is important to perform frequent screening of PDX and mouse colonies for both human and mouse pathogens, such as HIV and hepatitis for humans and Corynebacterium bovis for mice. This will limit the spreading of unwanted pathogens from the PDX to both the individual handling them and other mice in the study and vivarium.
The implantation methods described here can be used to study tumor biology, evaluate multiple aspects of the metastatic cascade, and for preclinical studies. The main advantage of flank implantation is the ease of tumor monitoring over time, as tumors are visible, and its growth can be easily measured using a caliper. This method can be a good place to start for establishing feasibility of a drug target. Intracranial implantation is preferred if the presence of the brain microenvironment is important and could alter the growth or molecular profile of the tumor. In addition, intracranial implantation places the tumor behind the blood brain barrier (BBB), making it essential for preclinical studies examining the efficacy of drugs required to traverse the BBB. However, it is difficult to monitor the growth of PDX tumors and requires radiological imaging or bioluminescent imaging if the cells are labeled. Knowing when to start the drug treatment preclinically would require either imaging data to monitor growth or knowledge of average survival of mice bearing a particular PDX tumor. Furthermore, intracranial implantation bypasses all the essential steps of the metastatic cascade, making it only suitable for studying drug efficacy and tumor microenvironment within the brain. Despite the differences in the tumor microenvironment, the morphology of PDX tumors is similar regardless of the site of implantation as can be seen in this PDX tumor (CM04) derived from a brain metastasis that originated from a small cell lung cancer primary tumor (Figure 2). The small cell lung cancer morphology of tumor cells with small nuclei and scant cytoplasm can be observed in the flank tumor, intracranial tumor, and abdominal metastasis resulting from intracardiac injection. Furthermore, spontaneous metastasis from tumors implanted in the flank have been previously observed12, suggesting that the metastatic processes such as intravasation, extravasation, and colonization can be recapitulated and studied in flank tumors which would otherwise not be possible with orthotopic tumors in the brain. In general, it is observed that flank implantation is a suitable method for studying CNS metastasis biology and conducting preclinical studies.
Intracardiac injection is most often used to study organ tropism and metastatic potential of tumors. Injected tumor cells would have to undergo several steps of the metastatic cascade, including surviving circulation, extravasation, and colonization of the metastatic site. Much like orthotopic injection into the brain, it may be difficult to track the progress of tumor metastasis without radiological imaging or cell labeling. However, as with orthotopic implantation, successful inoculation results in deterioration of the condition of the animals over time as the tumor spreads. Figure 3A demonstrates metastasis to the brain after intracardiac injection in CNS metastasis PDX model, M2, which originated from a melanoma. Intracardiac injection of PDX tumor (CM04) resulted in metastasis to the abdominal cavity and liver (Figure 3B). Other organs assessed, such as the lung, kidney, and ovaries had no visible metastases.

Figure 1: Flow chart showing the general workflow of establishing, propagating, and using PDX for preclinical studies. For each inoculation method, steps of the metastatic cascade involved are listed below each method. Please click here to view a larger version of this figure.
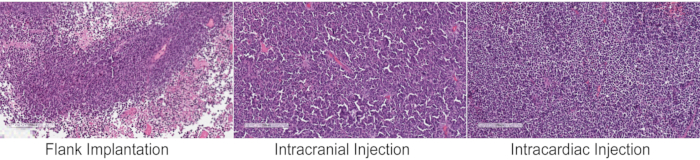
Figure 2: Histology of PDX tumors following different inoculation methods. Hematoxylin and eosin (H&E) staining of a CNS metastasis PDX tumor that originated from small cell lung cancer (CM04) implanted into immunocompromised mice by the three methods have similar tumor pathohistological and morphological features of small cell lung cancers, with small nuclei and scant cytoplasm. The intracardiac injection panel shows an abdominal metastasis. Nests of small-sized cells and high nuclear to cytoplasmic ratio is evident in all the three images. Images were taken on a slide scanner and magnified to 10x. Please click here to view a larger version of this figure.
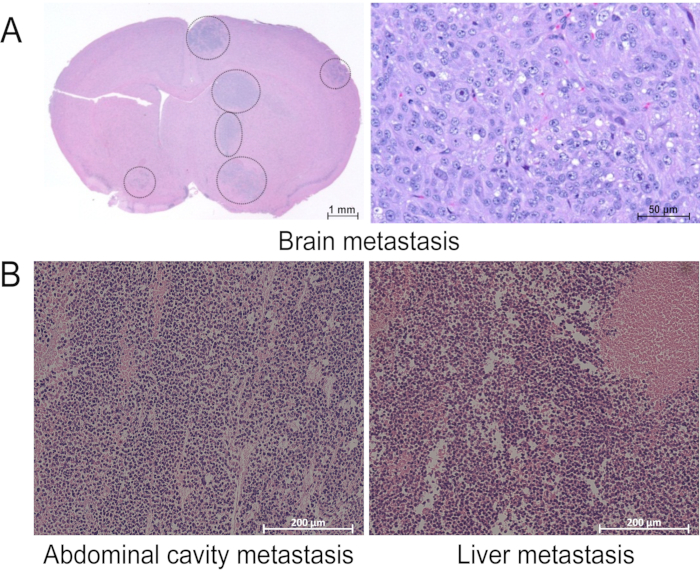
Figure 3: Metastases observed after intracardiac injection. H&E staining of tissues with visible metastasis during assessment by necropsy after intracardiac injection of (A) M2 and (B) CM04. Images were taken (A) on a slide scanner and magnified to 1x (left) or 20x (right) or (B) on a regular microscope at 10x magnification. This figure has been modified from our previous publication12. Please click here to view a larger version of this figure.
Discussion
In the current manuscript, methods for PDX establishment and propagation have been detailed. Three different inoculation methods that can be used for setting up preclinical studies when evaluating CNS metastasis have also been demonstrated. The method of choice should depend on the goals of the experiment. In some cases, it would be beneficial to use more than one inoculation route. For example, subcutaneous flank implantation provides a simple approach to study the effectiveness of a drug on tumor growth and assess the drug on its target and it also provides a visual for tumor size that is easily monitored and measured. However, once target feasibility and anti-tumor growth properties are established, one could set up an orthotopic study to assess the efficacy of the biologic to cross the BBB and study its effect within the brain tumor microenvironment. Also, survival is better assessed in orthotopic and intracardiac injection studies.
Intracranial injection of brain metastasis PDX models is often the preclinical model of choice due to the presence of the brain microenvironment and BBB. However, studies have shown that brain metastases have the ability to modify the BBB, which affects the permeability of molecules to the tumor16. These changes in the BBB would not be reflected by intracranially implanted tumors, and because of this preclinical drug studies may not fully reflect the response of patient tumors. Even with this caveat, intracranial injection remains the best method to test the permeability and efficacy of drugs to cross the BBB in preclinical models. Another challenge with intracranial models is that they are difficult for monitoring tumor growth and require the use of imaging techniques. Viral transduction of PDXs with fluorescent or bioluminescent markers has been traditionally used but can be challenging to perform. However, several imaging techniques are being developed for use in mice that do not require introduction of markers, which could improve the ease of monitoring these orthotopic brain tumors for preclinical studies. These include imaging technologies such as magnetic resonance imaging (MRI) and positron emission tomography (PET) imaging and micro computed tomography (micro-CT). Finally, intracranial injection may not accurately reflect the microenvironment of CNS metastases outside the brain, such as in leptomeningeal metastasis. In this case, injection into the cisterna magna could be performed to more accurately represent leptomeningeal metastases17.
Characterization of both the phenotypic and molecular features of the PDX model is important for selecting the best models for preclinical studies. Tumor latency can range from 7-140 days and take rates can also be highly variable12. The optimal number of animals to implant and timing to starting treatment would have to be based on the characteristics of each PDX model and needs to be empirically determined. Furthermore, the molecular profile of PDX tumors is also important for the selection of the most representative PDX models for preclinical studies. The closer the model molecularly represents the donor tissue the more predictive of clinical response it is likely to be. Also, it is critical to ensure that the targets selected from human data are present in the PDXs being chosen for studies and are sustained through a few generations, as shown clonal succession is associated with a different genomic profile of the incumbent dominant clone. In light of this, the phenotypic and molecular profiles of the CNS metastasis PDX tumors have been extensively characterized over multiple generations12.
Despite the many advantages of using CNS metastasis PDX models, there are several limitations related to their use. First, an alternate tumor microenvironment and especially lack of an immune system are well-documented limitations of PDX models18. The xenograft of a human tumor into mice results in the replacement of human stroma with mouse stroma with each subsequent passage and the human stroma is generally completely replaced after several passages19. However, the differences in the tumor microenvironment do not result in large differences in the molecular profile of flank-implanted PDX tumors compared to the original patient tumor12, suggesting that flank models still represent good experimental models to study CNS metastasis. Second, the use of immunocompromised animals results in a lack of immune cell infiltration in the tumor and a general immune response by the host, limiting a fundamental way in which the host attempts to combat cancer growth12. While humanized mice engrafted with human immune cells are available for studying the interactions of specific immune cells with the tumor, there are still many questions and controversies about the approaches, methods, and interpretation of those results20.
While the majority of PDXs have been shown to be genetically stable, we and others have shown that in rare cases, even in the absence of treatments or other external selective pressures, there could be changes in the clones of the tumors, such as minor clone takeover12,14,15. This could result in dramatic changes in the molecular profile, which would ultimately render the tumor not reflecting the dominant clones in the patient tumor12. While PDXs displaying clonal succession can have use in preclinical studies, many genes intended for targeting (e.g., Her2) could be lost with clonal succession. Therefore, frequent screening of PDX models to determine whether they still maintain the molecular profile of the desired clone is encouraged.
In summary, PDX models represent an excellent model system for the study of not only CNS metastasis but also other tumor types. Developing these models have shown that they largely reflect the phenotypic, molecular profile and heterogeneity of human CNS metastasis8,9,10,12. They serve as effective models for studying both CNS metastasis biology and also serve well as physiologically relevant preclinical models, replacing overused cell line models historically used for in vivo studies of CNS metastasis. Undoubtedly, differences between the PDX and donor patient tumor exists12,18. Knowing what these differences are is important for proper planning and execution of preclinical studies. Lastly, by choosing between several inoculation routes, PDX models are versatile in their use allowing the study of multiple aspects of the disease. PDXs models will no doubt play an important role in advancing our understanding of CNS metastasis and the development of novel therapies.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
Figure 3A was taken from our previous publication12 and was generated in Dr. Jann Sarkaria's lab at the Mayo Clinic.
Materials
| 25G needle | VWR | BD305122 | |
| 70 µm Cell strainer | VWR | 21008-952 | |
| 70% ethanol wipes | VWR | 470106-486 | |
| Bone wax | MedVet | W31G-RL | |
| CIEA NOG mouse | Taconic | NOG-F | |
| DMEM | ThermoFisher | 11965092 | |
| Ethiqa XR (buprenorphine SR) | MWI | 072117 | |
| FBS | ThermoFisher | 16000044 | |
| gentleMACS C Tube | Miltenyi | 130-093-237 | |
| gentleMACS Octo Dissociator | Miltenyi | 130-095-937 | |
| Hamilton syringe | Sigma | 20919 | |
| Matrigel growth factor reduced (GFR) | Corning | 354230 | |
| Ophthalmic ointment | MedVet | PH-PURALUBE-VET | |
| PBS/DPBS | ThermoFisher | 14040133 | |
| Povidone iodine swabs | VWR | 15648-906 | |
| Stereotaxic frame | Stoelting | 51730 | |
| Surgical drill | Stoelting | 58610 | |
| Surgical glue | MedVet | VG3 | |
| Surgical sutures | MedVet | MMV-661-V | |
| Syringe | VWR | 53548-001 | |
| Tumor dissociation kit | Miltenyi | 130-095-929 |
Referenzen
- Cruz-Munoz, W., Kerbel, R. S. Preclinical approaches to study the biology and treatment of brain metastases. Seminars in Cancer Biology. 21 (2), 123-130 (2011).
- Owonikoko, T. K., et al. Current approaches to the treatment of metastatic brain tumours. Nature Reviews. Clinical Oncology. 11 (4), 203-222 (2014).
- Salhia, B., et al. Integrated genomic and epigenomic analysis of breast cancer brain metastasis. PLoS One. 9 (1), 85448 (2014).
- Kotecha, R., Gondi, V., Ahluwalia, M. S., Brastianos, P. K., Mehta, M. P. Recent advances in managing brain metastasis. F1000Research. 7, 1000 (2018).
- DeRose, Y. S., et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nature Medicine. 17 (11), 1514-1520 (2011).
- Klinghammer, K., et al. A comprehensively characterized large panel of head and neck cancer patient-derived xenografts identifies the mTOR inhibitor everolimus as potential new treatment option. International Journal of Cancer. 136 (12), 2940-2948 (2015).
- Lee, H. W., et al. Patient-derived xenografts from non-small cell lung cancer brain metastases are valuable translational platforms for the development of personalized targeted therapy. Clincal Cancer Research: An Official journal of the American Association of Cancer Research. 21 (5), 1172-1182 (2015).
- Ni, J., et al. Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nature Medicine. 22 (7), 723-726 (2016).
- Oshi, M., et al. Novel breast cancer brain metastasis patient-derived orthotopic xenograft model for preclinical studies. Cancers (Basel). 12 (2), 444 (2020).
- Garman, B., et al. Genetic and genomic characterization of 462 melanoma patient-derived xenografts, tumor biopsies, and cell lines. Cell Reports. 21 (7), 1936-1952 (2017).
- Krepler, C., et al. A comprehensive patient-derived xenograft collection representing the heterogeneity of melanoma. Cell Reports. 21 (7), 1953-1967 (2017).
- Tew, B. Y., et al. Patient-derived xenografts of central nervous system metastasis reveal expansion of aggressive minor clones. Neuro-Oncology. 22 (1), 70-83 (2020).
- Liu, Z., et al. Improving orthotopic mouse models of patient-derived breast cancer brain metastases by a modified intracarotid injection method. Scientific Reports. 9 (1), 622 (2019).
- Davies, N. J., et al. Dynamic changes in clonal cytogenetic architecture during progression of chronic lymphocytic leukemia in patients and patient-derived murine xenografts. Oncotarget. 8 (27), 44749-44760 (2017).
- Eirew, P., et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature. 518 (7539), 422-426 (2015).
- Arvanitis, C. D., Ferraro, G. B., Jain, R. K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nature Reviews. Cancer. 20 (1), 26-41 (2020).
- Choi, S., et al. In vivo bioluminescence imaging for leptomeningeal dissemination of medulloblastoma in mouse models. BMC Cancer. 16 (1), 723 (2016).
- Hidalgo, M., et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discovery. 4 (9), 998-1013 (2014).
- Bradford, J. R., et al. Whole transcriptome profiling of patient-derived xenograft models as a tool to identify both tumor and stromal specific biomarkers. Oncotarget. 7 (15), 20773-20787 (2016).
- Yip, H., Haupt, C., Maresh, G., Zhang, X., Li, L. Humanized mice for immune checkpoint blockade in human solid tumors. American Journal of Clinical and Experimental Urology. 7 (5), 313-320 (2019).

