Synthesis of Functionalized Magnetic Nanoparticles, Their Conjugation with the Siderophore Feroxamine and its Evaluation for Bacteria Detection
Summary
This work describes protocols for the preparation of magnetic nanoparticles, its coating with SiO2, followed by its amine functionalization with (3-aminopropyl)triethoxysilane (APTES) and its conjugation with deferoxamine using a succinyl moiety as a linker. A deep structural characterization description and a capture bacteria assay using Y. enterocolitica for all the intermediate nanoparticles and the final conjugate are also described in detail.
Abstract
In the present work, the synthesis of magnetic nanoparticles, its coating with SiO2, followed by its amine functionalization with (3-aminopropyl)triethoxysilane (APTES) and its conjugation with deferoxamine, a siderophore recognized by Yersinia enterocolitica, using a succinyl moiety as a linker are described.
Magnetic nanoparticles (MNP) of magnetite (Fe3O4) were prepared by solvothermal method and coated with SiO2 (MNP@SiO2) using the Stöber process followed by functionalization with APTES (MNP@SiO2@NH2). Then, feroxamine was conjugated with the MNP@SiO2@NH2 by carbodiimide coupling to give MNP@SiO2@NH2@Fa. The morphology and properties of the conjugate and intermediates were examined by eight different methods including powder X-Ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM) and energy dispersive X-Ray (EDX) mapping. This exhaustive characterization confirmed the formation of the conjugate. Finally, in order to evaluate the capacity and specificity of the nanoparticles, they were tested in a capture bacteria assay using Yersinia enterocolitica.
Introduction
The bacteria detection methods using MNP are based on the molecular recognition of antibodies, aptamers, bioprotein, carbohydrates conjugated to MNP by the pathogenic bacteria1. Taking into account that siderophores are recognized by specific receptors on the outer membrane of bacteria, they could also linked to MNP to increase their specificity2. Siderophores are small organic molecules involved in the Fe3+ uptake by bacteria3,4. The preparation of conjugates between siderophores and MNP along with their evaluation for the capture and isolation of bacteria has not yet been reported.
One of the crucial steps in the synthesis of conjugates of magnetic nanoparticles with small molecules is the selection of the type of bond or interaction between them to ensure that the small molecule is attached to the surface of the MNP. For this reason, the procedure to prepare the conjugate between magnetic nanoparticles and feroxamine—the siderophore recognized by Yersinia enterocolitica—was focused at the generation of a modifiable surface of the MNP to allow linking it covalently to the siderophore by carbodiimide chemistry. In order to get an uniform magnetite nanoparticles (MNP) and to improve nucleation and size control, a solvolysis reaction with benzyl alcohol was carried in a thermal block without shaking5. Then, a silica coating was generated by Stöber method to confer protection and improve the stability of the nanoparticles suspension in aqueous media6. Taking into account the structure of the feroxamine, the introduction of amine groups is necessary to produce suitable nanoparticles (MNP@SiO2@NH2) to be conjugated with the siderophore. This was achieved by condensation of (3-aminopropyl)triethoxysilane (APTES) with the alcohol groups present on the surface of the silica modified nanoparticles (MNP@SiO2) using a sol-gel method7.
In parallel, the feroxamine iron(III) complex was prepared by complexation of the commercially available deferoxamine with iron acetyl acetonate in aqueous solution. N-succinylferoxamine, bearing succinyl groups that will act as linkers, was obtained by the reaction of feroxamine with succinic anhydride.
The conjugation between MNP@SiO2@NH2 and N-succinylferoxamine to give MNP@SiO2@NH@Fa was carried out through carbodiimide chemistry using as coupling reagents benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphonium hexafluorophosphate (BOP) and 1-hydroxybenzotriazole (HOBt) in a soft basic media to activate the terminal acid group in N-succinylferoxamine8.
Once the MNPs were characterized, we evaluated the capabilities of bare and functionalized magnetic nanoparticles to capture wild type (WC-A) and a mutant of Y. enterocolitica lacking feroxamine receptor FoxA (FoxA WC-A 12-8). Plain MNPs, functionalized MNPs and the conjugate MNP@SiO2@NH@Fa were allowed to interact with each Y. enterocolitica strain. The bacteria-conjugate aggregates were separated from the bacteria suspension by the application of a magnetic field. The separated aggregates were rinsed twice with phosphate buffered saline (PBS), re-suspended in PBS to prepare serial dilutions and then, they were plated for colony counting. This protocol demonstrates each step of the synthesis of MNP@SiO2@NH@Fa, the structural characterization of all the intermediates and the conjugate, and a bacterium capture assay as an easy way to evaluate the specificity of the conjugate in relation to the intermediates.9
Protocol
NOTE: For the reactions performed under inert atmosphere conditions, all the glassware was previously dried in an oven at 65 °C, sealed with a rubber septum and purged with argon three times.
1. Synthesis of magnetic nanoparticles conjugated with feroxamine
- Synthesis of Fe3O4 magnetic nanoparticles (MNPs)
- Add 0.5 g of Fe(acac)3 in a 20 mL glass vial and then mix with 10 mL of benzyl alcohol.
- Sonicate this mixture for 2 min, then transfer to a heating block and heat at 180 °C for 72 h.
- After the reaction is completed, allow the vials to cool down, rinse the nanoparticles with 96% ethanol and centrifuge at 4000 x g for 30 min. Repeat the centrifugation at least twice.
- Separate the nanoparticles from the supernatant by magnetic attraction using a neodymium (NdFeB) magnet and discard the residual solvent.
- Rinse with 96% ethanol repeating step 1.1.4. and discard the supernatant alternating with sonication in a bath for 1 min at 40 kHz until the solvent looks clear.
- Magnetic nanoparticles SiO2 coating (MNP@SiO2)
- Prepare a suspension of 2 g of MNP in 80 mL of isopropanol and then add 4 mL of 21% ammonia, 7.5 mL of distilled water and 0.56 mL of tetraethyl orthosilicate (TEOS) (in this order) in a round bottom flask with a magnetic stir bar.
- Heat the mixture at 40 °C for 2 h with continuous stirring and then sonicate for 1 h.
- Separate the MNP with a magnet, discard the supernatant, and disperse it in 30 mL of isopropanol.
- Repeat steps 1.2.1. and 1.2.2.
- Remove and wash the magnetic stir bar with 96% ethanol to recover all the material.
- Separate the nanoparticles from the supernatant by magnetic attraction using a magnet.
- Discard the supernatant and rinse the nanoparticles with 96% ethanol three times alternating with sonication.
- Dry the nanoparticles under vacuum at room temperature for 12 h.
- Functionalization of MNP@SiO2 with (3-aminopropyl)triethoxysilane (APTES)
- Rinse 500 mg of the MNP@SiO2 obtained from the previous step with N,N-dimethylformamide (DMF) under inert atmosphere and then sonicate for 1 min at 40 kHz. Then, discard the supernatant and repeat this process three times.
- Re-suspend the particles in a round bottom flask, under stirring with a magnetic stir bar and add 9 mL of APTES.
- Stir the mixture at 60 °C for 12 h.
- Discard the supernatant and rinse the nanoparticles with 96% ethanol three times alternating with sonication.
- Synthesis of feroxamine
- Dissolve 100 mg (0.15 mmol) of deferoxamine mesylate salt and 53.0 mg (0.15 mmol) of Fe(acac)3 in 5 mL of distilled water and stir the mixture overnight at room temperature.
- Wash the resulting product three times with 20 mL of EtOAc in a separation funnel and then, remove the organic solvent under vacuum using a rotatory evaporator.
- Freeze-dry the aqueous phase to afford feroxamine as a red solid.
- Synthesis of N-succinylferoxamine
- Add 350 mg (3.50 mmol) of succinic anhydride to a solution of 100 mg (0.17 mmol) of feroxamine in 5 mL of pyridine in a 50 mL round bottomed flask under inert atmosphere.
- Stir the resulting mixture at room temperature for 16 h. After that time, remove the excess of pyridine under reduced pressure in a rotatory evaporator to give a dark red solid.
- Dissolve the reaction crude in 3 mL of methanol.
- Transfer the methanolic solution into a Sephadex column (20 cm of Sephadex in a 20 mm diameter column) and elute at 0.5 mL/min.
- Collect the red fraction and remove the methanol under vacuum using a rotatory evaporator.
- Synthesis of the conjugate MNP@SiO2@NH@Fa
- Rinse 30 mg of dry MNP@SiO2@NH2 twice with DMF and sonicate the nanoparticles in a 100 mL Erlenmeyer flask for 30 min under inert atmosphere.
- Prepare a solution of N-succinylferoxamine (200 mg, 0.30 mmol), benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphonium hexafluorophosphate (BOP, 173 mg, 0.45 mmol), 1-hydroxybenzotriazole (HOBt, 46 mg, 0.39 mmol) and N,N-diisopropylethylamine (DIPEA, 128.8 mg, 1.21 mmol) in 10 mL of DMF (Mix A) in a 50 mL round-bottomed flask under inert atmosphere.
- Suspend the previously rinsed MNP@SiO2@NH2 in 3 mL of DMF under sonication in dry under oxygen free conditions using an argon gas atmosphere (Mix B).
- Add mix A to mix B dropwise.
- Shake the final mixture using an orbital shaker at room temperature overnight.
- Separate the resulting conjugate (MNP@SiO2@NH@Fa) from the suspension using a magnet.
- Rinse the resulting solid and then, sonicate it five times with 10 mL of ethanol.
- Dry the solid under vacuum for 24 h.
2. Bacterial assay with Y. enterocolitica strains to quantify the capture of pathogenic bacteria with nanoparticles
- Prepare a suspension of all the intermediate nanoparticles and the final conjugate in PBS at 1 mg/mL in sterile 2 mL tubes.
- Prepare a culture of Y. enterocolitica in 5 mL of Luria Bertani (LB) broth overnight incubating at 37 °C.
- Prepare a 5 mL of iron deficient tryptic soy broth (TSB) by adding 50 µL of 10 mM 2,2′-bipyridyl.
- Inoculate the 5 mL of iron deficient TSB with 50 µL of the overnight culture of Y. enterocolitica and then, incubate at 37 °C with agitation until an OD600 = 0.5‒0.8 is reached.
- Take 100 µL of the culture obtained in the step 2.4 and dilute in a 2.0 mL tube containing 900 µL of PBS to obtain a first 1/10 dilution. Then, prepare a 1/100 dilution from the first dilution using the same procedure to get a concentration of bacterial cells at 1 x 106 Colony Forming Units (CFU)/mL approximately.
- Add 100 µL of nanoparticles suspension at 1 mg/mL to 1 mL of the 1/100 dilution of bacterial suspension in a 2.0 mL tube, and homogenize with vortex.
- Incubate the culture at 20 °C for 1 h.
- Separate the MNP/bacteria aggregates by using a magnet and carefully discard the supernatant.
- Rinse the separated nanoparticles twice with 1 mL PBS using a vortex.
- Suspend the nanoparticles in 1 mL of PBS to count the amount of bacterial capture in CFU/mL.
- Prepare four successive 1/10 dilutions from the former suspension until a 1 x 10-4 dilution is reached.
- Plate 10 µL of each dilution onto TS agar plates and incubate them at 37 °C overnight.
- Photograph the plate with a gel digitalizer in epi white mode. Process the image with an appropriate software to amplify a spot to count the number of individual colonies.
NOTE: Each MNP intermediate was characterized to follow up the progress of the synthesis. Firstly, bare MNPs were studied by XRD to check the crystalline structure. Then, the FT-IR spectrum of each intermediate was run to check the changes that occurred in the corresponding reaction. Raman spectroscopy analysis of each intermediate was also carried out in order to confirm the conclusions deduced from FT-IR spectra. TGA analysis allowed us to estimate the loss weight of the intermediates bearing organic material in its structure. The morphology and size of each intermediate was studied by TEM. Finally, XPS analysis was critical to determine the atom oxidation states at each MNP intermediate surface and to confirm the covalent bond formation in the conjugate MNP@SiO2@NH@Fa.
Representative Results
An exhaustive structural characterization is carried out in order to determine the morphology and the properties of each intermediate and the final conjugate. For this purpose, the techniques XRD, FT-IR, Raman spectroscopy, TGA, TEM, EDX mapping and XPS are used in order to demonstrate the formation of the conjugate. The oxidation states of the atoms at the surface of the nanoparticles acquired by X-ray photoelectron spectroscopy (XPS) are the most relevant data to confirm the formation of covalent bonds between the nanoparticle and the siderophore. In agreement with these results, this protocol is reproducible.
Bare MNP, functionalized MNPs and the conjugate are mixed with each Y. enterocolitica strain in PBS solution. The bacteria-MNPs aggregates are separated from the suspension using a magnet. After rinsing the aggregates twice with PBS, they are re-suspended in PBS to prepare serial dilutions that are plated for colony counting.
Intermediates and the final conjugate prepared using this protocol were submitted to several techniques to display the changes that take place in each step of the synthesis. Infrared and Raman spectroscopy constitute an easy and fast way to monitor each step of the synthesis. The presence of characteristic bands corresponding to Si-O, C-Si-C, Fe-O, O=C amide vibration, O=C-N hydroxamic acid vibration in the FT-IR and Raman (see below) spectra were the first indicators of the chemical changes that were taking place on the surface of the magnetic nanoparticles in each step of the synthesis.
XRD diffractogram
Figure 1 shows the XRD analysis used to confirm the composition and crystalline structure of the synthetic magnetic nanoparticles (MNP) of magnetite by comparison with JCPDS file 00-003-0863.
TEM analysis
Figure 2C displays bright spots of the electron diffraction pattern that match with the (111), (220), (311), (400), (422), (511) and (440) diffraction planes of magnetite corresponding to d-spacings of 4.9, 2.9, 2.4, 2.0, 1.7, 1.6 and 1.4 Å, respectively. On the other hand, Figure 2D and Figure 4E show TEM images of MNP@SiO2@NH2@Fa corresponding to dispersed MNP particles (~10 nm) embedded in the amorphous inorganic-organic material. The coating thickness is over ~10 nm.
EDX analysis
EDX maps display the distribution of the elements Fe, O, Si and C on the surface. Figure 3A clearly shows the presence of Si on the surface of MNP@SiO2. After amine functionalization and conjugation with feroxamine, the increment of C on the surface of nanoparticles for MNP@SiO2@NH@Fa is shown in Figure 3B as an evidence of a successful conjugation.
IR analysis
FTIR spectra of bare MNP, MNP@SiO2, MNP@SiO2@NH2 and MNP@SiO2@NH@Fa is shown in Figure 4. All the FTIR spectra display the beginning of a band within the spectral range of the analysis at 600 cm-1 which was related to Fe–O vibrations. The presence of a broad band at 1050 cm-1—attributed to the Si-O-Si stretching vibration—in the FTIR spectra of MNP@SiO2, MNP@SiO2@NH2 and MNP@SiO2@NH@Fa confirmed the silica coating.
The FTIR spectrum of MNP@SiO2 (Figure 4) displays a broad band between at 830 and 1275 cm-1 (Si-O bond); it becomes more intense after its functionalization with APTES in the FTIR spectrum of MNP@SiO2@NH2, probably due to the Si-C bond (expected between 1175 and 1250 cm-1). Finally, the bands at 2995 cm-1 (C-H stretching bonds), 1640 cm-1 (O=C-NH amide II vibration) and 1577 cm-1 (O=C-N hydroxamic acid vibration) observed in the FTIR spectrum of MNP@SiO2@NH@Fa confirmed the conjugation of feroxamine with the nanoparticles10.
Thermogravimetry analysis
Thermogravimetry data is shown in Figure 5, which displays the weight loss due to addition of organic material and water.
Raman analysis
The silica coating and functionalization of the bare MNP were also confirmed by Raman analysis of each intermediate and MNP@SiO2@NH@Fa (Figure 6). All Raman spectra show peaks at 305.8, 537.2 and 665.6 cm-1, corresponding to Fe-O vibrations (Figure 6A)11, and a shoulder on the peak at 713.5 cm-1, relating to Si-O-Si vibrations (Figure 6B)12. After APTES functionalization, the Raman spectrum of MNP@SiO2@NH2 displays intense peaks at 1001.5 and 1027.4 cm-1, corresponding to the presence of SiO2, and at 1578.6 and 1597.9 cm-1, confirming the formation of Si-C bonds. Furthermore, the presence of a shoulder of the peak at 703.0 cm-1 also confirmed the presence of APTES (Figure 6C)13,14. Finally, the broad peak between 1490 and 1700 cm-1 (centred at ~1581 cm-1), corresponding to Si-C bonds and amide groups in the Raman spectrum of MNP@SiO2@NH@Fa, are in agreement with the formation of the conjugate (Figure 6D)14.
XPS analysis
The study of the oxidation state of the atoms on the surface was performed by XPS analysis and confirmed the bonds formation in the structures. Figure 7 shows the XPS spectra of the bare and different functionalized MNPs. For MNP, a narrow peak in C1s might be due to impurities in handling the sample during the synthesis. The introduction of carbon is observed as C-C and C-H bonds in MNP@SiO2@NH2 and MNP@SiO2@NH@Fa spectra. The analysis of the peak at 399 eV in the N1s spectra along with its attenuation observed for MNP@SiO2@NH@Fa confirms the formation of amide bonds between the MNP@SiO2@NH2 and feroxamine. Moreover, the existence of N-O bond of hydroxamic moieties is in agreeement with the presence of a peak at 402 eV. The presence of a peak at 102 eV in Si2p narrow spectra in all intermediates and the conjugate is in agreement with the binding energy for the siloxane group15,16.
Z Potential
Z potential values are displayed In Table 1. The results are negative, -25.21 and -29.35 mV, for MNP and MNP@SiO2, respectively. Functionalization with APTES to give MNP@SiO2@NH2 changed the surface charge from negative to positive. This fact was attributed to the amine groups and the surface charge remains positive for MNP@SiO2@NH@Fa. The positive surface Z potential could explain the interaction between bacteria (whose surface is negative) and the conjugate17,18,19.
Bacteria capture assay
The number of cells of Y. enterocolitica WC-A and FoxA WC-A 12-8 captured with MNP intermediates and MNP@SiO2@NH@Fa were quantified in those dilutions where 40‒60 colonies were separated and easily visualized. The number of captured cells by bare, MNP@SiO2, and MNP@SiO2@NH2 shows no significant differences among them (Figure 8). The electrostatic forces due to free amine groups in MNP@SiO2@NH2 and the low concentration of feroxamine membrane receptor in bacteria might justify the lack of expected binding specificity.
This protocol could be applied in the synthesis of different type of conjugates, mainly those that use carbodiimide chemistry. It is versatile enough to introduce modifications in order to obtain better results. The complete characterization of all the intermediates and the final conjugate, using the described techniques, allows one to follow each step of the synthesis and confirm the formation of the desire bond. The counting of colonies in the bacteria capture assay, using a 10 µL drop, allows one to test all the samples at the same time in one plate which makes easier to get replicates and perform assays under different conditions.
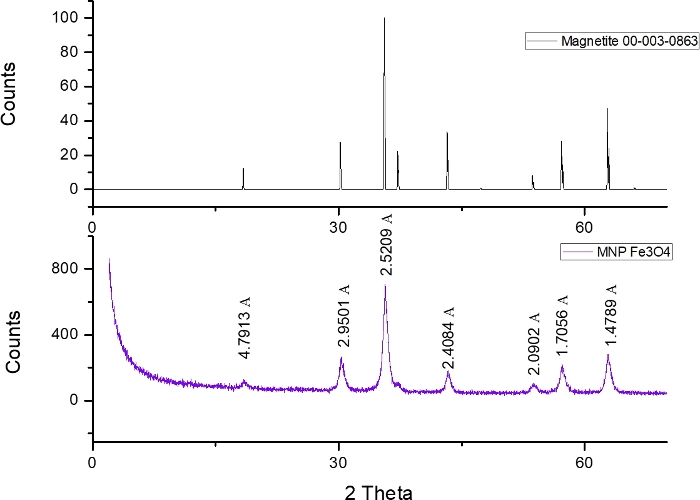
Figure 1: Comparison of MNP (Fe3O4) (purple) and magnetite pattern (black) diffractograms.
This figure has been modified from Martínez-Matamoros et al.9. Please click here to view a larger version of this figure.
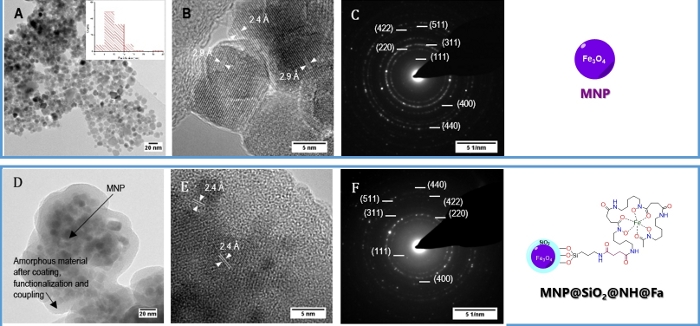
Figure 2: Bright field TEM and electron diffraction images of bare MNP (A, B and C), and of MNP@SiO2@NH@Fa (D, E and F).
Images are at medium and high resolutions. This figure has been modified from Martínez-Matamoros et al.9. Please click here to view a larger version of this figure.
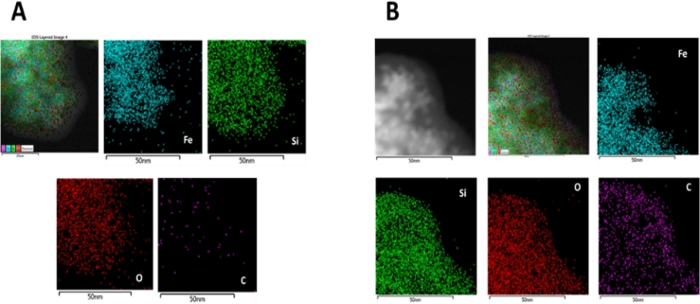
Figure 3: EDX maps of MNP@SiO2: HAADF image and the corresponding Fe, Si, O and C maps of A. MNP@SiO2 and B. MNP@SiO2@NH@Fa.
This figure has been modified from Martínez-Matamoros et al.9. Please click here to view a larger version of this figure.
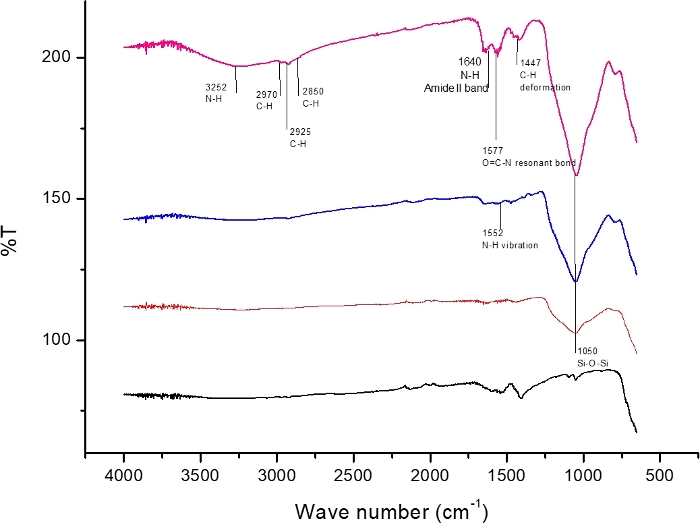
Figure 4: FT-IR spectra of bare iron oxide (Fe3O4) MNP, MNP@SiO2, MNP@SiO2@NH2 and MNP@SiO2@NH@Fa (4).
This figure has been modified from Martínez-Matamoros et al.9. Please click here to view a larger version of this figure.
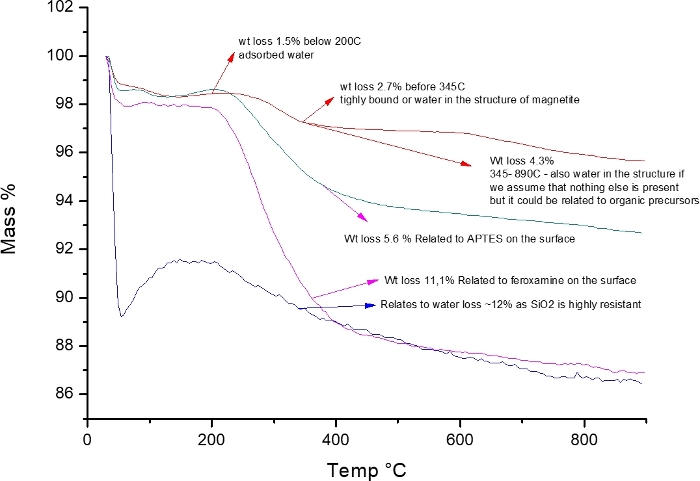
Figure 5: Thermogravimetric analysis of MNP, MNP@SiO2@NH2, and MNP@SiO2@NH@Fa.
This figure has been modified from Martínez-Matamoros et al.9. Please click here to view a larger version of this figure.
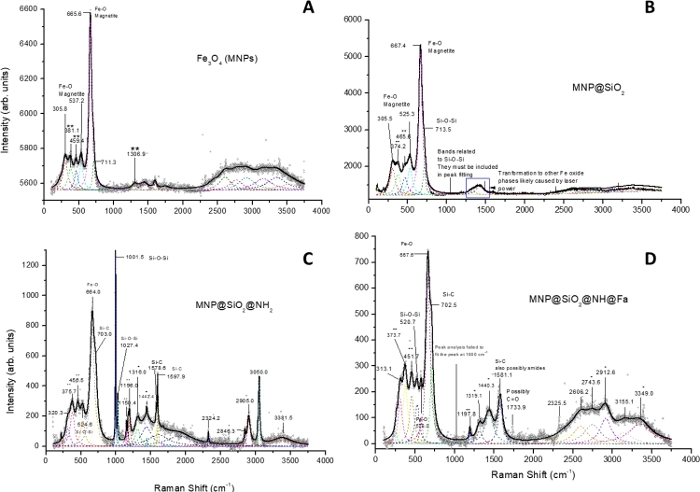
Figure 6: Raman spectra of bare iron oxide (Fe3O4) MNP (A), MNP@SiO2 (B), MNP@SiO2@NH2 (C) and MNP@SiO2@NH@Fa (D).
(*) APTES, (**) Other iron oxide phases, likely formed from the transformation of magnetite by the laser power. This figure has been modified from Martínez-Matamoros et al.9. Please click here to view a larger version of this figure.
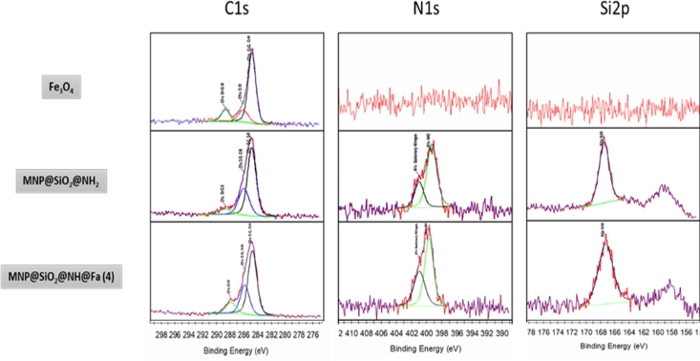
Figure 7: XPS narrow spectra of MNP, MNP@SiO2@NH2 and MNP@SiO2@NH@Fa. This figure has been modified from Martínez-Matamoros et al.9. Please click here to view a larger version of this figure.
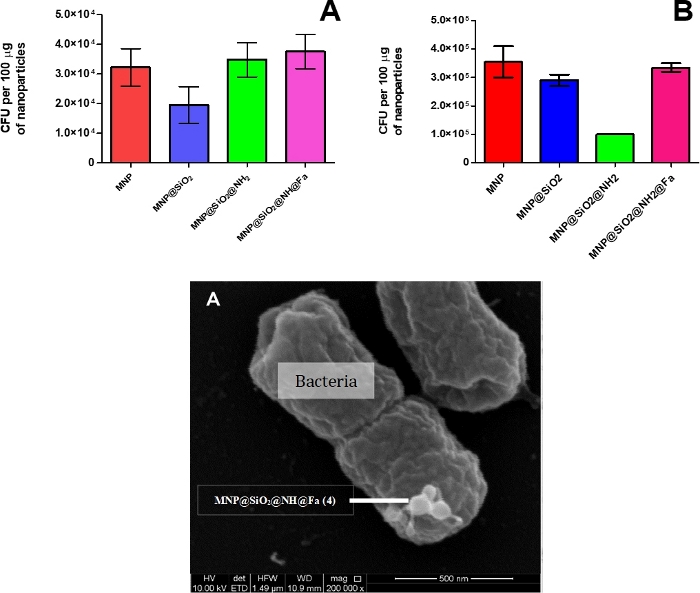
Figure 8: CFU of Y. enterocolitica captured per 100 µg of magnetic nanoparticles: bare, MNP@SiO2, MNP@SiO2@NH2 and MNP@SiO2@NH@Fa.
(A) WC-A (wild type) (B) FoxA WC-A 12-8 (mutant lacking feroxamine receptor FoxA) and SEM image of MNP@SiO2@NH@Fa interacting with Y. enterocolitica. This figure has been modified from Martínez-Matamoros et al.9. Please click here to view a larger version of this figure.
| Sample | Z Potential |
| MNP | -25.21 |
| MNP@SiO2 | -29.35 |
| MNP@SiO2@NH2 | 17.03 |
| MNP@SiO2@NH@Fa | 22.14 |
| MNP@SiO2@NHBoc@Fa | 19.16 |
| MNP@SiO2@NHCOOH@Fa | 10.96 |
Table 1: Z potential measurements. This table has been obtained from Martínez-Matamoros et al.9.
Discussion
This protocol describes the synthesis of a conjugate between magnetic nanoparticles and the siderophore feroxamine by covalent bonding. The synthesis of magnetite was carried out using the protocol reported by Pinna et al.5 followed by silica coating to protect the magnetic core of corrosion in aqueous systems, to minimize the aggregation and to provide a suitable surface for functionalization6. The silica coating process was modified. Instead of carrying out three coatings as reported by Li et al.6, the MNPs were coated with two silica layers in this method (Figure 2D) which was enough to continue with the functionalization step with APTES7.
Deferoxamine was complexed with iron (III) because it is susceptible to degradation within a few hours at room temperature. The best way to obtain the iron complex is using iron acetyl acetonate because its purification by liquid-liquid extraction is very simple and feroxamine is obtained in a quantitative yield. Feroxamine is stable and could be modified to give N-succinylferoxamine using succinic anhydride to add a terminal acidic group as a linker. The purification by size exclusion chromatography allows removal of the excess succinic anhydride and succinic acid from N-succinylferoxamine.
The amine nanocomposite was used to link N-succinylferoxamine covalently on the surface. Nuclear magnetic resonance (NMR) cannot be used for the structural elucidation of the products due to the paramagnetic behavior of iron oxide. For this reason, each reaction step was monitored by FT-IR and then confirmed by Raman spectroscopy. Peaks deconvolution and fitting were made with a convenient software taking into account that the Raman spectra included ten measures of 300 s with a total measure time of 3,000 s. Morphology and size were measured by TEM observing the pseudospherical shape magnetite with a homogeneous size distribution of 10 nm (Figure 2A,B). The conjugate coating thickness measure show a 10 nm homogeneous layer (Figure 2D,E).
It is very difficult to obtain a TEM field where nanoparticles can be observed individually due to its magnetic character and its tendency to agglomerate. EDX mapping was used to obtain information on the composition of each element on the surface (Figure 3A). The carbon density was clearly increased in the conjugate MNP@SiO2@NH@Fa (Figure 3B) relative to that of the intermediate MNP@SiO2.
The bacteria capture assay was designed in order to test the capability of the conjugate to capture bacteria through the feroxamine outer membrane protein receptor. Yersinia enterocolitica WC-A strain was selected because it expresses the FoxA feroxamine receptor and is easy to grow. The critical step in this procedure was the removal of non-captured bacteria. This was achieved by rinsing and vortexing with sterile PBS twice followed by recovering the bacteria-conjugate aggregates using a magnet. The numbers of captured cells by each of the MNP intermediate, used as control, and the conjugate MNP@SiO2@NH@Fa were determined by colony counting from dilution method. Using 10 µL drops facilitates the experimental procedure and allows to handle more than four samples reducing the cost of the test in terms of time and material as compared to the classical method of plaque counting colonies.
The most important limitation of the synthesis of the MNP@SiO2@NH@Fa conjugate is that it is not possible to confirm the bond formation by NMR. Although the preparation of MNP@SiO2@NH@Fa seems simple, the use of the structural characterization techniques is crucial in order to confirm the linking between the siderophore and the MNP through a covalent bond.
Following the present protocol, it is possible to generate conjugates using carbodiimide chemistry. In order to achieve this, the presence of amino groups on the MNPs surface and a carboxylic acid functionality in the compound of interest are necessary. Testing different linkers and coating the MNPs surface with other functional groups to avoid creating positive charges on it could improve the bacterial capture discrimination.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors gratefully acknowledge Professor Klaus Hantke (University of Tübingen, Germany) for kindly supply the Yersinia enterocolitica strains used in this work. This work was supported by grants AGL2015-63740-C2-1/2-R and RTI2018-093634-B-C21/C22 (AEI/FEDER, EU) from the State Agency for Research (AEI) of Spain, co-funded by the FEDER Programme from the European Union. Work in University of Santiago de Compostela and University of A Coruña was also supported by grants GRC2018/018, GRC2018/039, and ED431E 2018/03 (CICA-INIBIC strategic group) from Xunta de Galicia. Finally, we want to thank to Nuria Calvo for her great collaboration doing the voice-off this video protocol.
Materials
| 1-Hydroxybenzotriazole hydrate HOBT |
Acros | 300561000 | |
| 2,2′-Bipyridyl | Sigma Aldrich | D216305 | |
| 3-Aminopropyltriethoxysilane 99% | Acros | 151081000 | |
| Ammonium hydroxide solution 28% NH3 | Sigma Aldrich | 338818 | |
| Benzotriazol-1-yloxytris(dimethylamino)-phosphonium hexafluorophosphate BOP Reagent | Acros | 209800050 | |
| Benzyl alcohol | Sigma Aldrich | 822259 | |
| Deferoxamine mesylate salt >92,5% (TLC) | Sigma Aldrich | D9533 | |
| Ethanol, anhydrous, 96% | Panreac | 131085 | |
| Ethyl Acetate, Extra Pure, SLR, Fisher Chemical | |||
| Iron(III) acetylacetonate 97% | Sigma Aldrich | F300 | |
| LB Broth (Lennox) | Sigma Aldrich | L3022 | |
| N,N-Diisopropylethylamine, 99.5+%, AcroSeal | Acros | 459591000 | |
| N,N-Dimethylformamide, 99.8%, Extra Dry, AcroSeal | Acros | 326871000 | |
| Pyridine, 99.5%, Extra Dry, AcroSeal | Acros | 339421000 | |
| Sephadex LH-20 | Sigma Aldrich | LH20100 | |
| Succinic anhydride >99% | Sigma Aldrich | 239690 | |
| Tetraethyl orthosolicate >99,0% | Sigma Aldrich | 86578 |
Referenzen
- Pan, Y., Du, X., Zhao, F., Xu, B. Magnetic nanoparticles for the manipulation of proteins and cells. Chemical Society Reviews. 41 (7), 2912-2942 (2012).
- Zheng, T., Nolan, E. M. Siderophore-based detection of Fe(III) and microbial pathogens. Metallomics. 4, 866-880 (2012).
- Hider, R. C., Kong, X. Chemistry and biology of siderophores. Natural Product Reports. 27 (5), 637-657 (2010).
- Sandy, M., Butler, A. Microbial Iron Acquisition: Marine and Terrestrial Siderophores. Chemical Reviews. 109 (10), 4580-4595 (2010).
- Pinna, N., Grancharov, S., Beato, P., Bonville, P., Antonietti, M., Niederberger, M. Magnetite Nanocrystals : Nonaqueous Synthesis, Characterization. Chemistry of Materials. 17 (15), 3044-3049 (2005).
- Li, Y. S., Church, J. S., Woodhead, A. L., Moussa, F. Preparation and characterization of silica coated iron oxide magnetic nano-particles. Spectrochimica Acta – Part A: Molecular and Biomolecular Spectroscopy. 76 (5), 484-489 (2010).
- Chen, J. P., Yang, P. C., Ma, Y. H., Tu, S. J., Lu, Y. J. Targeted delivery of tissue plasminogen activator by binding to silica-coated magnetic nanoparticle. International Journal of Nanomedicine. 7, 5137-5149 (2012).
- El-Boubbou, K., Gruden, C., Huang, X. Magnetic glyco-nanoparticles: a unique tool for rapid pathogen detection, decontamination, and strain differentiation. Journal of the American Chemical Society. 129 (44), 13392-13393 (2007).
- Martínez-Matamoros, D., et al. Preparation of functionalized magnetic nanoparticles conjugated with feroxamine and their evaluation for pathogen detection. RSC Advances. 9 (24), 13533-13542 (2019).
- Cozar, O., et al. Raman and surface-enhanced Raman study of desferrioxamine B and its Fe(III) complex, ferrioxamine B. Journal of Molecular Structure. 788 (1-3), 1-6 (2006).
- Shebanova, O. N., Lazor, P. Characterisation of a-C:H and oxygen-containing Si:C:H films by Raman spectroscopy and XPS. Journal of Solid State Chemistry. 174 (4), 424-430 (2003).
- González, P., Serra, J., Liste, S., Chiussi, S., León, B., Pérez-Amor, M. Raman spectroscopic study of bioactive silica based glasses. Journal of Non-Crystalline Solids. 320 (12), 92-99 (2003).
- Veres, M., et al. Characterisation of a-C:H and oxygen-containing Si:C:H films by Raman spectroscopy and XPS. Diamond and Related Materials. 14 (3-7), 1051-1056 (2005).
- You, Y., et al. Visualization and investigation of Si-C covalent bonding of single carbon nanotube grown on silicon substrate. Applied Physics Letters. 93 (10), 103111-103113 (2008).
- Graf, N., et al. XPS and NEXAFS studies of aliphatic and aromatic amine species on functionalized surfaces. Surface Science. 603 (18), 2849-2860 (2009).
- Michaeli, W., Blomfield, C. J., Short, R. D., Jones, F. R., Alexander, M. R. A study of HMDSO/O2 plasma deposits using a high-sensitivity and -energy resolution XPS instrument: curve fitting of the Si 2p core level. Applied Surface Science. 137 (1-4), 179-183 (2002).
- Liana, A. E., Marquis, C. P., Gunawan, C., Gooding, J. J., Amal, R. T4 bacteriophage conjugated magnetic particles for E. coli capturing: Influence of bacteriophage loading, temperature and tryptone. Colloids and Surfaces B: Biointerfaces. 151, 47-57 (2017).
- Fang, W., Han, C., Zhang, H., Wei, W., Liu, R., Shen, Y. Preparation of amino-functionalized magnetic nanoparticles for enhancement of bacterial capture efficiency. RSC Advances. 6, 67875-67882 (2016).
- Zhan, S., et al. Efficient removal of pathogenic bacteria and viruses by multifunctional amine-modified magnetic nanoparticles. Journal of Hazardous Materials. 274, 115-123 (2014).

