A Static Self-Directed Method for Generating Brain Organoids from Human Embryonic Stem Cells
Summary
This protocol was generated as a means to produce brain organoids in a simplified, low cost manner without exogenous growth factors or basement membrane matrix while still maintaining the diversity of brain cell types and many features of cellular organization.
Abstract
Human brain organoids differentiated from embryonic stem cells offer the unique opportunity to study complicated interactions of multiple cell types in a three-dimensional system. Here we present a relatively straightforward and inexpensive method that yields brain organoids. In this protocol human pluripotent stem cells are broken into small clusters instead of single cells and grown in basic media without a heterologous basement membrane matrix or exogenous growth factors, allowing the intrinsic developmental cues to shape the organoid's growth. This simple system produces a diversity of brain cell types including glial and microglial cells, stem cells, and neurons of the forebrain, midbrain, and hindbrain. Organoids generated from this protocol also display hallmarks of appropriate temporal and spatial organization demonstrated by brightfield images, histology, immunofluorescence and real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR). Because these organoids contain cell types from various parts of the brain, they can be utilized for studying a multitude of diseases. For example, in a recent paper we demonstrated the use of organoids generated from this protocol for studying the effects of hypoxia on the human brain. This approach can be used to investigate an array of otherwise difficult to study conditions such as neurodevelopmental handicaps, genetic disorders, and neurologic diseases.
Introduction
Due to myriad practical and ethical limitations, there has been a great deal of difficulty in studying the human brain. While studies utilizing rodents have been critical to our understanding of the human brain, the mouse brain has many dissimilarities1,2. Interestingly, mice have a neuronal density that is at least 7 times less than the primate brain3,4. Although primates are closer to humans than rodents from an evolutionary standpoint, it is not practical for most researchers to work with them. The purpose of this protocol was to recapitulate many important features of the human brain using a simplified and less expensive method without the need for a heterologous basement membrane matrix or exogenous growth factors while maintaining brain cell diversity and cellular organization.
Formative work from the Sasai lab used the serum free culture of embryoid bodies (SFEBq) method to generate two- and three- dimensional neuronal cell types from signalized embryonic stem cells (ESCs)5,6. Many human brain organoid methods have followed a relatively similar path from signalized ESCs7,8. In contrast, this protocol starts with clusters of detached human ESCs (hESCs), similar to the initial steps of seminal work of the Thomson and Zhang laboratories prior to the plating steps9,10 as well as the initial step of the brain organoid protocol of the Pasca laboratory before the addition of exogenous growth factors11. Basement membrane matrices (e.g., matrigel) have been utilized in many brain organoid protocols and it has been shown to be an effective scaffold8. However, most commonly used basement membrane matrices do not come without complications as they co-purify with unknown quantities of growth factors with batch to batch variability during production12. In addition, these matrices can complicate imaging, and increase the risk of contamination and cost.
While human brain organoids can be used to answer many questions, there are certain limitations to bear in mind. For one, starting from embryonic stem cells, organoids more closely resemble immature brains than aged brains and as such may not be ideal models for diseases that occur in old age, like Alzheimer's disease. Second, while our protocol found markers of forebrain, midbrain and hindbrain development which are useful to study the effect of a treatment or disease on cells from multiple brain regions in concert, other protocols can be followed to concentrate on specific brain regions13,14. Finally, another limitation of organoid models is that of size, while the average length of a human brain approximately 167 mm, brain organoids made with the use of agitation grow up to 4 mm8 and the organoids formed by this protocol grow to 1-2 mm by 10 weeks. Nonetheless, this protocol provides an important tool to study human brain tissue and the interaction of multiple cell types.
Protocol
1. Stem Cell Maintenance
- Maintain H9 hESCs on a layer of growth factor reduced basement membrane matrix (see the Table of Materials, henceforth simply referred to as matrix) according to the manufacturer's instructions.
- To coat one 6-well plate or one 10 cm dish, combine 100 µL of matrix with 5.9 mL of ice-cold Dulbecco's modified Eagle medium (DMEM)/F12 media. Wrap plates in paraffin film and store overnight at 4 °C. Use them on the next day for passaging cells after the excess matrix/media is aspirated.
- Culture the cells week to week at approximately a 1:12 split ratio every 7 days. Maintain the cells using mTESR-1 media in a 37 °C, low oxygen incubator (5% O2, 5% CO2). Refresh media daily. Weed out differentiating cells from the culture between passages using glass tools.
- H9 cells should be passaged four to six days prior to utilizing them to produce organoids. The cells should be passaged at approximately a 1:8 ratio of cell clusters. To do this, start by rinsing the cells with DMEM F12 media and dissociate the cells with a neutral protease (e.g., dispase, henceforth referred to simply as protease), rinse with DMEM/F-12, and plate as 30-60 cell clusters across 4 plates (6-well or 10 cm) at ~20% confluency. Two days prior to harvest, transition them to a regular incubator (21% O2, 5% CO2). Plates should reach ~80% confluency when starting organoid formation.
2. Dissociation of the hESCs for Organoid Culture
- Aliquot the protease stock solution (5 U/mL).
NOTE: We typically freeze down 1 mL aliquots at -20 °C for use over several months. - Dilute the protease stock solution to the working concentration by adding 1 mL of the stock solution plus 5 mL of DMEM/F12 for each 6-well or 10 cm plate of hESCs.
- Aspirate and remove cell culture media, then cover the hESCs with the protease solution. Place plates in the incubator for 10-15 min or until the edges of the colonies round up and begin to separate from the matrix.
- Tilt the plate, aspirate the protease solution, and gently wash the cells with DMEM/F12 three times. Use 2 mL/well for each wash when using a 6-well plate and 6 mL when using a 10 cm plate. Make sure colonies stay attached to the matrix when performing this step.
- Add back about 1.5 mL of fresh mTESR media to each well (or 5 mL for a 10 cm plate) and flush the cells off the plate using gentle pipetting.
- Using a 10 mL pipette, gently aspirate and dispense hESC within the plate until they reach approximately 1/30th of their original size. Colony clusters should resemble ~250-350 µm sized squares at the completion of these steps.
3. Generation of Organoids
- Transfer cells into a single ultra-low attachment T75 flask containing 30 mL of mTESR media without basic fibroblast growth factor (bFGF).
- The next day, tilt the flask(s) such that the live cells pool in the corner (this may take 5-10 min on the first day, but will get quicker as the clusters get larger).
NOTE: If there are a large number of cells that have adhered to the bottom of the flask at this step or any subsequent steps, transfer the cells to a new flask. It is normal to have a high population of dead cells for the first two days. When performing media changes, be sure to remove as much of the cell debris as possible. - Once the cells settle, aspirate off the media and dead cells leaving about 10 mL of media containing the live cells.
- Add ~20 mL of low bFGF media (DMEM/F12 supplemented with 1x N2, 1x B27, 1x L-glutamine, 1x NEAA, 0.05% bovine serum albumin (BSA), and 0.1 mM monothioglycerol (MTG) supplemented with 30 ng/mL bFGF).
- Check the cells on day 2. If most of the cells look healthy and bright, there is no need to do anything. However, if more than a third of the cells appear dark, replace the media (using the same tilting technique as in step 3.2) with ~20 mL of low bFGF media supplemented with 20 ng/mL bFGF.
- On day 3, replace half of the media (using the tilting technique in step 3.2) with 20 mL of low bFGF media supplemented with 10 ng/mL bFGF.
- On day 5, replace half of the medium (using the tilting technique in step 3.2) with 20 mL of neural induction media (NIM: DMEM/F12, 1x N2 supplement, 0.1 mM MEM NEAA, 2 µg/mL heparin).
NOTE: If there are any large clusters of cells or organoids that are much larger than the others, they should be removed from the culture. Size is estimated by appearance under the microscope; for example, using an eyepiece with reticle. The majority of organoids are similarly sized (roughly 100 ± 20 µm). We removed organoids that were approximately 2x smaller or larger than the others. - Replace half of the medium (~15 mL) (using the tilting technique) with NIM every other day.
- After 3 weeks in culture, add 100x penicillin/streptomycin to the media (NIM: DMEM/F12, 1x N2 supplement, 0.1 mM MEM NEAA, 2 µg/mL heparin) at a final concentration of 1x if desired. Refresh the media every other day.
NOTE: In this fashion, we maintained the organoids for up to 6 months in culture.
4. RNA Extraction and Preparation
- Gently extract approximately 15 organoids (depending upon size) from the flask using a 10 mL pipette and place into a 1.5 mL tube.
- Gently pellet the organoids in the centrifuge (200 x g for 1 min), and rinse with 1x Dulbecco's PBS (DPBS) three times.
- Extract RNA using validated system or protocol (e.g., RNeasy kit).
- Measure the optical density value of each sample at 260 and 280 nm.
- Prepare cDNA using a validated system or protocol (e.g., iScript cDNA synthesis kit).
- Perform qRT-PCR using pre-validated primers (Table 1) including at least one housekeeping gene.
5. Immunohistochemistry
- Fixation
- Prepare a 4% paraformaldehyde (PFA) solution and place it at 4 °C.
- Using a sterile razor, cut the tip off of a sterile transfer pipette.
- Gently extract organoids using the cut transfer pipette, as they can be easily broken apart, especially when they grow large, and place them into a 6-well plate with additional media or DPBS.
- Tilt the plate, aspirate the media, and replace with 1x DPBS. Rinse the cells with 1x DPBS two additional times.
- Replace the DPBS with 4% PFA solution and place on a shaker at 4 °C.
NOTE: While we fixed for 2 days (for small organoids) to 7 days (for organoids >3 months), shorter times (e.g., 16-24 h) may also be possible. - Prepare 30%, 20% and 10% sucrose solutions in DPBS.
- After fixation in PFA, replace with the 10% sucrose solution and place on a shaker at 4 °C for 24 h.
- Replace the 10% sucrose with 20% sucrose and place on a shaker at 4 °C for 24 h.
- Replace the 20% sucrose with 30% sucrose and place on a shaker at 4 °C for 24 h.
- Frozen sections
- Prepare a flat layer of dry ice and place a labeled plastic mold on top of it.
- Pour a thin layer of optimal cutting temperature medium (OCT) into the mold and let it start to harden (within a few seconds).
- Place a few organoids on the top of the OCT in the mold using a transfer pipette with the tip cut off and pay close attention to the location of the organoids.
- Slowly add in OCT until the mold is full and the organoids are covered. Let it harden completely for an additional 10 min.
NOTE: While freezing over 10 min helps ensure ideal relative placement of multiple organoids for sectioning, it is possible to use an ethanol-dry ice mix or liquid nitrogen to freeze more quickly. - Mark the relative location of the organoids with a marker to make it easier to find them when cutting.
- Place the molds in a bag or box and store at -80 °C until ready to cut the sections.
- Using a cryostat, slice 10 µm sections and place the tissue onto labeled, positively charged slides.
- Staining
- Prepare blocking solution (0.3% Triton X-100, 4% normal donkey serum in PBS).
- Use a hydrophobic pap pen to draw around the perimeter of the tissue.
- Rinse the slides with PBS 3 times for 5 min each.
- Replace PBS with blocking solution for 1 h at room temperature.
- Replace the blocking solution with antibody solution (antibody at appropriate concentration, 0.1% Triton X-100, 4% normal donkey serum in PBS) at 4 °C overnight.
- On the following day, wash the slide 3 times with PBS for 10 min each.
- Replace PBS with the appropriate secondary antibody (at the appropriate concentration) diluted in antibody solution for 1 h at room temperature.
- Rinse 3 times for 10 min each time with 1x PBS.
- Apply the 4',6-diamidino-2-phenylindole (DAPI) stain and rinse three times for 10 min each with 1x PBS.
- Affix coverslips to the front of the slides with mounting solution, let dry at room temperature in the dark, and store in the dark at 4 °C.
Representative Results
Figure 1 shows representative brightfield images of several time points to demonstrate what the cells/organoids look like throughout the different stages of the protocol. The hESCs were removed from the tissue culture plate, broken into small pieces, and placed in a T75 ultra-low attachment flask where they formed spheres. It is important to note that the cells look bright and similar in size, without dark, dying cells in the centers of these clusters. The cells were gradually weaned off bFGF. On day 5, they were placed into neural induction media and they remained in this media throughout the culture period. Although the organoids get larger and thus darker over time, it is important to take note of the neural rosette-like structures (black arrows) that are present throughout the brain organoid development and expand. The rosettes indicate the initiation of neural differentiation and contain features of the embryonic neural tube, displaying epithelial characteristics and surrounding an apical lumen15.
Staining of the organoids with hematoxylin and eosin at 5 months in culture indicated that there were not vast amounts of necrosis even in the centers, which was of initial concern given the stagnant culture system (Figure 2A). These organoids demonstrated a histologic morphology similar to the human cortex based on light microscopic evaluation by an experienced neuropathologist (Figure 2B). By histology, many unique cell morphologies were observed resembling glia (blue arrow head), neurons (red arrowhead), cells with Cajal-Retzius morphology (black arrows), and neuropil (orange arrow head) (Figure 2B,C).
To take a more in depth look at gene expression within the cells, qRT-PCR was performed. For the results shown in Figure 3, each bar represents 3 separate batches of cells grown independently and harvested at the specified time point. These samples were then run in triplicate with a primer pair to the indicated gene in addition to the housekeeping gene, GAPDH. The glutamate transporter, Vglut1 (Figure 3A), was expressed at 2.5 weeks, increased at 5 weeks, and remained consistent through 5 months in culture. A forebrain marker, Foxg1 (Figure 3B), was expressed at low levels until 5 weeks in culture. The deep layer marker, Tbr1 (Figure 3C), peaked around 5 weeks and decreased subsequently, whereas the upper layer marker, Satb2 (Figure 3D), increased over time.
The expression of the ventral marker Engrailed1 (Eng1) (Figure 3E), the hindbrain/spinal cord marker Hoxb4 (Figure 3F), as well as the oligodendrocyte marker, Olig2 (Figure 3G), all increased over time. In contrast, the stem cell marker, Sox2 (Figure 3H), decreased over time. The glial marker, FAP (Figure 3I), peaked at 5 weeks and remained relatively constant subsequently. In addition, immunofluorescence data was consistent with the qRT-PCR data. At 10 weeks there was a robust expression of Foxg1 (Figure 4A). Sox2 expression was more confined to areas resembling the subventricular zone (SVZ) (Figure 4B,C). Interestingly, there was also some expression of the outer radial glial cell marker, HopX (Figure 4D).
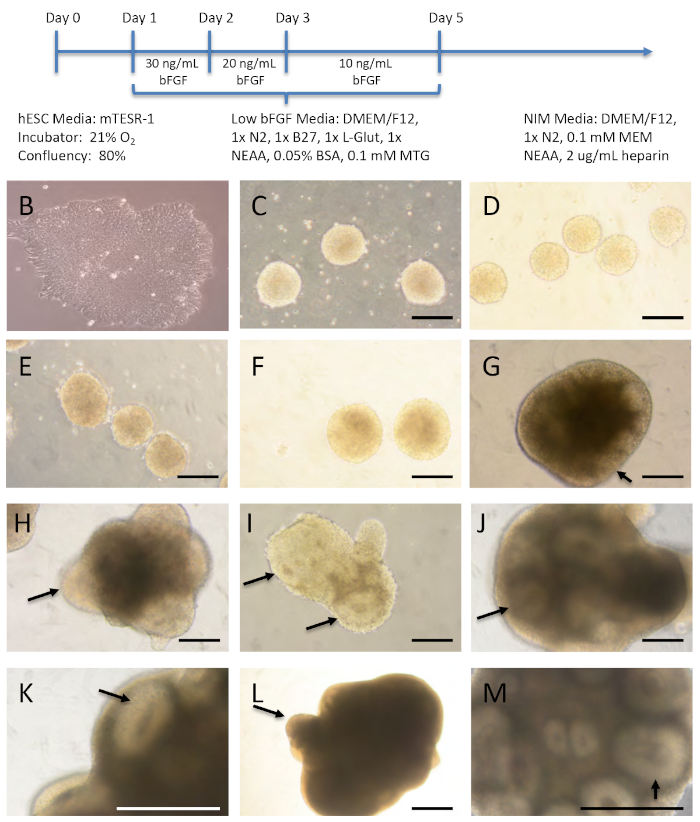
Figure 1: Overview of organoid growth conditions and morphology. (A) Schematic of media changes. (B-M) Representative images of organoids as they matured. (B-M) H9 hESCs (B) were utilized to form the brain organoids. Organoids on (C) day 2 in 20 ng/mL bFGF media, and (D) day 3 and (E) day 4 in 10 ng/mL bFGF media. (F-M) Organoids in neural induction media (NIM) on days 5 (F), 8 (G), 10 (H), 17 (I) 35 (J,K), and 70 (L,M). Arrows point to neural rosettes. Scale bar = 200 µm. Please click here to view a larger version of this figure.
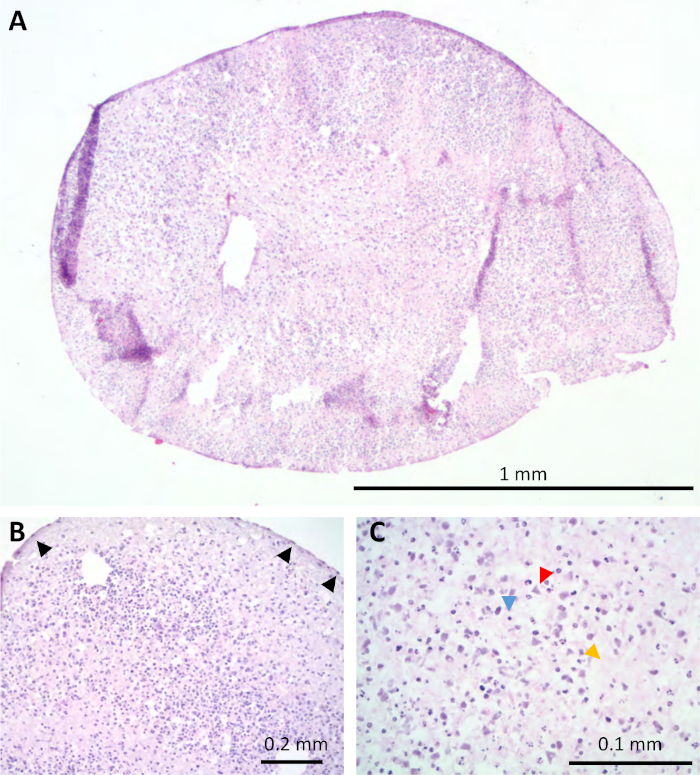
Figure 2: Organoids shared histologic similarities to human brain tissue. H&E staining of the organoids at 5 months (A) with some layering resembling the human cortex (B). At higher magnification many cell morphologies were observed including glia (blue arrow head), neurons (red arrowhead), neuropil (orange arrow head), and cells with Cajal-Retzius morphology (black arrows) (B,C). Please click here to view a larger version of this figure.
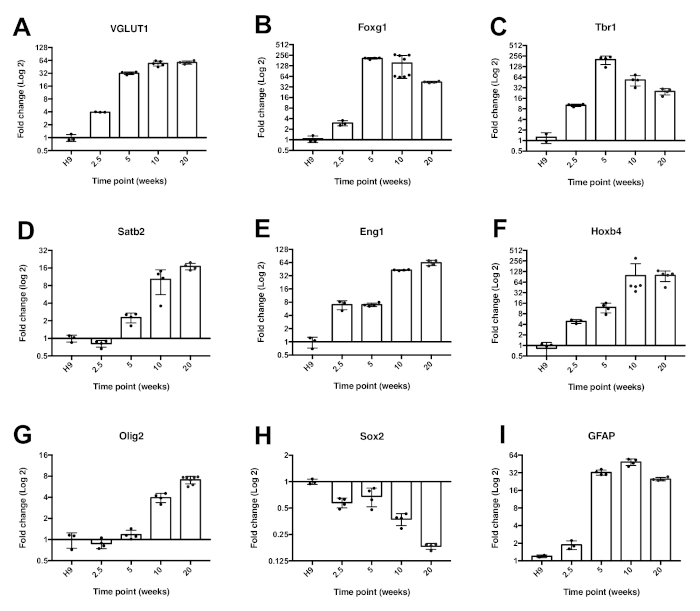
Figure 3: Expression of neurodevelopmental genes within the brain organoids over time. Quantitative RT-PCR data using SYBR green evaluating the expression of Vglut1 (A), Foxg1 (B), Tbr1 (C), Satb2 (D), En1 (E), Hoxb4 (F), Olig2 (G), Sox2 (H), and GFAP (I). Error bars = mean ± standard deviation (n ≥ 3). This figure has been modified from16. See Table 1 for primer information. Please click here to view a larger version of this figure.
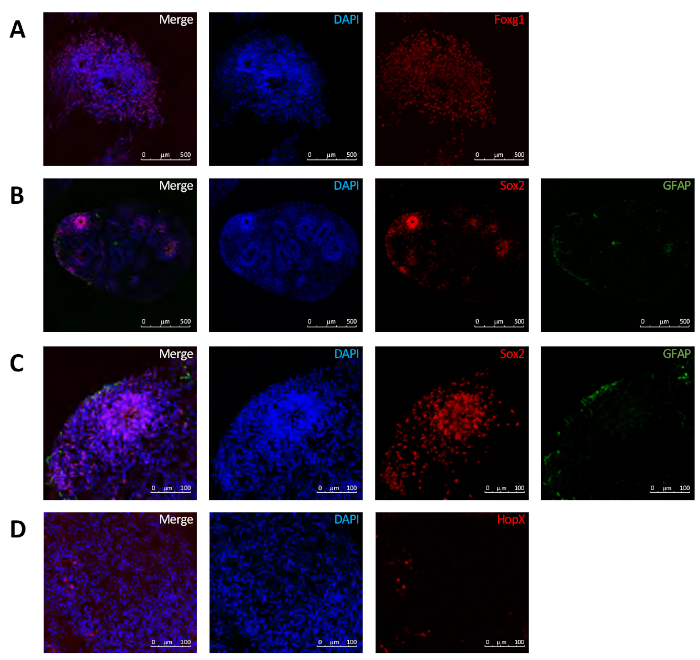
Figure 4: Expression of neurodevelopmental proteins within the brain organoids at 10 weeks. Immunofluorescence revealed a robust expression of Foxg1 (A), localized expression of Sox2 (B,C) and the presence of HopX (D). Please click here to view a larger version of this figure.
| Gene | Sequence (5' to 3') | Amplicon | Exon | |
| En1 | F | GGACAATGACGTTGAAA CGCAGCA |
149 | 2 |
| R | AAGGTCGTAAGCGGTTT GGCTAGA |
2 | ||
| Foxg1 | F | AGAAGAACGGCAAGTAC GAGA |
188 | 1 |
| R | TGTTGAGGGACAGATTG TGGC |
1 | ||
| GAPDH | F | ACCACAGTCCATGCCAT CAC |
449 | 8 |
| R | CACCACCCTGTTGCTGT AGCC |
9 | ||
| GFAP | F | AGAGATCCGCACGCAGT ATG |
80 | 4 |
| R | TCTGCAAACTTGGAGCG GTA |
5-Apr | ||
| Hoxb4 | F | AAAGCACCCTCTGACTG CCAGATA |
80 | 2 |
| R | ATGGGCACGAAAGATGA GGGAGA |
2 | ||
| Olig2 | F | CCCTGAGGCTTTTCGGA GCG |
451 | 1 |
| R | GCGGCTGTTGATCTTGA GACGC |
2 | ||
| Satb2 | F | TAGCCAAAGAATGCCCT CTC |
94 | 6 |
| R | AAACTCCTGGCACTTGG TTG |
7 | ||
| Sox2 | F | CCCAGCAGACTTCACAT GT |
150 | 1 |
| R | CCTCCCATTTCCCTCGT TTT |
1 | ||
| Tbr1 | F | GTCACCGCCTACCAGAA CAC |
101 | 4 |
| R | ACAGCCGGTGTAGATCG TG |
6 | ||
| Vglut1 | F | CAGAGTTTTCGGCTTTG CTATTG |
183 | 5-Apr |
| R | GCGACTCCGTTCTAAGG GTG |
6 |
Table 1: Primer sequences used for quantitative RT-PCR in Figure 3.
Discussion
Similar to other organoid models, this is an artificial system that comes with several caveats. Although there was little batch to batch variation in terms of overall expression levels, individual organoids did exhibit differences. For example, the location of Sox-2 positive areas were not identical in every organoid (Figure 3). While qPCR is suitable to look for overall changes in batches of cells, additional techniques such as single cell RNAseq will be utilized in future studies to gather more information on a cell-by-cell basis. Another limitation of this system, is that it does not integrate vasculature within the organoids as has been done in some of the more recent studies17,18,19. However, transitioning the hESCs from a low to high oxygen environment may more closely resemble the anaerobic to aerobic transitioning in a developing embryo.
The critical steps within this protocol are the formation of the neurospheres as well as the appropriate maintenance including media changes with the proper culture media to ensure healthy cells and adequate nutrients to the growing organoids without overcrowding. To troubleshoot inadequate cell proliferation or differentiation, we recommend starting with a fresh batch of low passage ESCs and freshly prepared media including supplements. Occasionally there can be batch variation of reagents and materials. Thus, we recommend purchasing multiple bottles of reagents such as N2 and ultra-low attachment flasks which are from the same lot as long as they can be utilized in a reasonable amount of time.
Unlike many other brain organoid protocols, this method does not use a bioreactor; instead the cells stay relatively stagnant aside from media changes. This is similar to previous work with neurospheres, which were eventually broken apart to make 2D neuronal cultures20. In this model the cells are kept in a 3D format and allowed to grow for up to 6 months in culture. It was found that when utilizing small clusters instead of aggregating single cells that the organoids looked brighter, which we interpreted as less necrotic. As previously reported, when the brain organoid clusters were evaluated by histology and immunofluorescence at 5 months, there were no obvious areas of necrosis16. Although starting from small clusters of cells introduces a little variety in the size of organoids formed, the majority of organoids were of a roughly similar size.
The use of a heterologous basement membrane matrix and a bioreactor have both advantages and disadvantages. Certain cell types, or larger brain organoids might prefer growth under one condition or another. Basement membrane matrices or other hydrogels might be beneficial to selectively add growth factors to particular regions or create specific molds. Although basement membrane matrices have been shown to support three-dimensional organization and differentiation15, it is worth emphasizing that some of these products have a poorly defined and variable composition that includes quantities of growth factors12. In addition to simplifying the workflow while culturing the brain organoids, the absence of a basement membrane matrix might also improve three-dimensional imaging techniques.
The development of this brain organoid model system offers a new approach for many potential applications. For example, toxic insults like hypoxia, hyperglycemia, hypercapnia, and infection among others, may be tested with this system. In addition, neurodevelopmental disorders may be studied with this system by starting with either genetically modified stem cells or patient-specific human induced pluripotent stem cells (hPSCs). The ability to add different cell types during organoid culture also offers the possibility to study tumor-brain interactions. Given the simplicity of the protocol and lack of expensive, specialty materials we hope that this approach may be considered by laboratories both within and outside of the field as one potential method with its own unique benefits to further advance this rapidly progressing and exciting discipline.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We thank the Yale Stem Cell Core (YSCC), and the Yale Cancer Center (YCC) for assistance. We thank Dr. Jung Kim for his neuropathology review. This work was supported by Connecticut Regenerative Medicine Research Fund, March of Dimes, and NHLBI R01HL131793 (S.G.K.), the Yale Cancer Center and the Yale Cancer Biology Training Program NCI CA193200 (E.B.) and a generous unrestricted gift from Joseph and Lucille Madri.
Materials
| Alexa Fluor 488 goat anti-mouse | Thermo Fisher Scientific, Waltham, MA, USA | A11029 | |
| Alexa Fluor 546 goat anti-rabbit | Thermo Fisher Scientific, Waltham, MA, USA | A11035 | |
| B27 Supplement | Gibco, Waltham, MA, USA | 17504-044 | |
| bFGF | Life Technologies, Carlsbad, CA, USA | PHG0263 | |
| BSA | Sigma-Aldrich, St. Louis, MO, USA | A9647 | |
| BX43 microscope | Olympus, Shinjuku, Tokyo, Japan | ||
| DAPI stain | Thermo Fisher Scientific, Waltham, MA, USA | D1306 | |
| Dispase | STEMCELL Technologies, Vancouver, Canada | 07913 | |
| DMEM/F12 | Thermo Fisher Scientific, Waltham, MA, USA | 11330-032 | |
| DPBS | Gibco, Waltham, MA, USA | 10010023 | |
| FluroSave | MilliporeSigma, Burlington, MA | 345789 | |
| GFAP antibody | NeuroMab, Davis, CA | N206A/8 | |
| Growth Factor Reduced Matrigel (Matrix) | Corning, Corning, NY, USA | 356231 | |
| H9 hESCs | WiCell, Madison, WI, USA | WA09 | |
| Heparin | Sigma-Aldrich, St. Louis, MO, USA | 9041-08-1 | |
| iQ SYBR Green Supermix | Bio-Rad, Hercules, CA, USA | 1708880 | |
| iScript cDNA Synthesis Kit | Bio-Rad, Hercules, CA, USA | 1708891 | |
| L-glutamine | Gibco, Waltham, MA, USA | 25030-081 | |
| Monothioglycerol | Sigma-Aldrich, St. Louis, MO, USA | M6145 | |
| mTESR media | STEMCELL Technologies, Vancouver, Canada | 85850 | |
| N2 NeuroPlex | Gemini Bio Products, West Sacramento, CA, USA | 400-163 | |
| Nanodrop | Thermo Fisher Scientific, Waltham, MA, USA | ND-2000 | |
| NEAA | Gibco, Waltham, MA, USA | 11140-050 | |
| Normal Donkey Serum (NDS) | ImmunoResearch Laboratories Inc., West Grove, PA, USA | 017-000-121 | |
| OCT | Sakura Finetek, Torrance, CA, USA | 25608-930 | |
| PFA | Electron Microscopy Sciences, Hatfield, PA | RT15710 | |
| qPCR machine | Bio-Rad, CFX96, Hercules, CA, USA | 1855196 | |
| RNeasy kit | Qiagen, Hilden, Germany | 74104 | |
| Sox2 | MilliporeSigma, Burlington, MA | AB5603 | |
| TMS-F microscope | Nikon, Melville, NY, USA | ||
| Triton X-100 | Sigma-Aldrich, St. Louis, MO, USA | T8787-100ML | |
| Ultra-low attachment T75 flasks | Corning, Corning, NY, USA | 3814 |
Referenzen
- Northcutt, R. G. Understanding vertebrate brain evolution. Integrative and Comparative Biology. 42 (4), 743-756 (2002).
- Roth, G. . The Long Evolution of Brains and Minds. , (2013).
- Herculano-Houzel, S. The human brain in numbers: a linearly scaled-up primate brain. Frontiers in Human Neuroscience. 3, 31 (2009).
- Roth, G., Dicke, U. Evolution of the brain and intelligence. Trends in Cognitive Sciences. 9 (5), 250-257 (2005).
- Eiraku, M., et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 3 (5), 519-532 (2008).
- Watanabe, K., et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nature Neuroscience. 8 (3), 288-296 (2005).
- Kadoshima, T., et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proceedings of the National Academy of Sciences of the United States of America. 110 (50), 20284-20289 (2013).
- Lancaster, M. A., et al. Cerebral organoids model human brain development and microcephaly. Nature. 501 (7467), 373-379 (2013).
- Li, X. J., et al. Specification of motoneurons from human embryonic stem cells. Nature Biotechnology. 23 (2), 215-221 (2005).
- Zhang, S. C., Wernig, M., Duncan, I. D., Brustle, O., Thomson, J. A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nature Biotechnology. 19 (12), 1129-1133 (2001).
- Pasca, A. M., et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 12 (7), 671-678 (2015).
- Hughes, C. S., Postovit, L. M., Lajoie, G. A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 10 (9), 1886-1890 (2010).
- Qian, X., et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 165 (5), 1238-1254 (2016).
- Sloan, S. A., Andersen, J., Pasca, A. M., Birey, F., Pasca, S. P. Generation and assembly of human brain region-specific three-dimensional cultures. Nature Protocols. 13 (9), 2062-2085 (2018).
- Kelava, I., Lancaster, M. A. Stem Cell Models of Human Brain Development. Cell Stem Cell. 18 (6), 736-748 (2016).
- Boisvert, E. M., Means, R. E., Michaud, M., Madri, J. A., Katz, S. G. Minocycline mitigates the effect of neonatal hypoxic insult on human brain organoids. Cell Death and Disease. 10 (4), 325 (2019).
- Bergmann, S., et al. Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nature Protocols. 13 (12), 2827-2843 (2018).
- Mansour, A. A., et al. An in vivo model of functional and vascularized human brain organoids. Nature Biotechnology. 36 (5), 432-441 (2018).
- Pham, M. T., et al. Generation of human vascularized brain organoids. Neuroreport. 29 (7), 588-593 (2018).
- Boisvert, E. M., Denton, K., Lei, L., Li, X. J. The specification of telencephalic glutamatergic neurons from human pluripotent stem cells. Journal of Visualized Experiments. (74), (2013).

