Electroretinogram Recording in Larval Zebrafish using A Novel Cone-Shaped Sponge-tip Electrode
Summary
Here, we present a protocol that simplifies the measurement of light evoked electroretinogram responses from larval zebrafish. A novel cone-shaped sponge-tip electrode can help to make the study of visual development in larval zebrafish using the electroretinogram ERG easier to achieve with reliable outcomes and lower cost.
Abstract
The zebrafish (Danio rerio) is commonly used as a vertebrate model in developmental studies and is particularly suitable for visual neuroscience. For functional measurements of visual performance, electroretinography (ERG) is an ideal non-invasive method, which has been well established in higher vertebrate species. This approach is increasingly being used for examining the visual function in zebrafish, including during the early developmental larval stages. However, the most commonly used recording electrode for larval zebrafish ERG to date is the glass micropipette electrode, which requires specialized equipment for its manufacture, presenting a challenge for laboratories with limited resources. Here, we present a larval zebrafish ERG protocol using a cone-shaped sponge-tip electrode. The novel electrode is easier to manufacture and handle, more economical, and less likely to damage the larval eye than the glass micropipette. Like previously published ERG methods, the current protocol can assess outer retinal function through photoreceptor and bipolar cell responses, the a- and b-wave, respectively. The protocol can clearly illustrate the refinement of visual function throughout the early development of zebrafish larvae, supporting the utility, sensitivity, and reliability of the novel electrode. The simplified electrode is particularly useful when establishing a new ERG system or modifying existing small-animal ERG apparatus for zebrafish measurement, aiding researchers in the visual neurosciences to use the zebrafish model organism.
Introduction
The zebrafish (Danio rerio) has become a widely used genetic vertebrate model, including studies of the visual neurosciences. The increasing popularity of this species can be attributed to advantages including ease of genetic manipulation, the highly conserved vertebrate visual system (neuron types, anatomical morphology and organization, and underlying genetics), high fecundity and lower cost of husbandry compared to mammalian models1. The non-invasive electroretinogram (ERG) has long been used clinically to assess human visual function, and in the laboratory setting to quantify vision in a range of large and small species including rodents and larval zebrafish2,3,4,5. The most commonly analyzed ERG components are the a-wave and b-wave, originating from the light-sensing photoreceptors and bipolar interneurons, respectively. In larval zebrafish, distinct layers in the retina are established by 3 days post-fertilization (dpf) and the morphology of the photoreceptor cone terminal synapses mature before 4 dpf6,7. Outer retinal function of larval zebrafish is thus established before 4 dpf, meaning that the ERG is measurable from this early age onwards. Because of the short experimental cycle and the high-throughput properties of the model, the ERG has been applied to larval zebrafish for functional assessment of disease models, analyzing color vision and retinal development, studying visual circadian rhythms and testing drugs8,9,10,11,12.
However, current approaches for larval zebrafish ERG has some complexities that may make it harder to adopt. Published larval zebrafish ERG protocols commonly use a glass micropipette filled with conductive liquid as the recording electrode3,4,5,13, which requires a high quality micropipette tip3. Specialized equipment, such as a micropipette puller and in some cases a microforge, are required for their manufacture. This can be a challenge for laboratories with limited resources and leads to extra costs even when adapting available small animal ERG systems for measurement of larval zebrafish visual function. Even when smoothed, the sharp micropipette tip can damage the surface of the larval eye. Additionally, commercial micropipette holders for electrophysiology are constructed with a fixed silver wire. These fixed wires become passivated after repetitive use, requiring the purchase of new holders leading to increased maintenance costs.
Here we describe an ERG method using a cone-shaped sponge-tip recording electrode, that is particularly useful for adapting established small-animal ERG setups for larval zebrafish ERG measurements. The electrode is easily made using common polyvinyl acetate (PVA) sponge and fine silver wire without any other specialized equipment. Our data show that this novel electrode is sensitive and reliable enough to demonstrate the functional development of retinal neural circuits in larval zebrafish between 4 and 7 dpf. This economical and practical sponge-tip electrode may be useful to researchers establishing new ERG systems or modifying existing small-animal systems, for zebrafish studies.
Protocol
All electroretinogram (ERG) procedures were performed according to the provisions of the Australian National Health and Medical Research Council code of practice for the care and use of animals and were approved by the institutional animal ethics committee at the University of Melbourne.
1. Buffer Preparation
- Prepare the 10x goldfish Ringer’s buffer (1.25 M NaCl, 26 mM KCl, 25 mm CaCl2, 10 mM MgCl2, 100 mM glucose, 100 mM HEPES) using reverse osmosis (RO) water. Adjust the buffer to pH 7.8 and sterilize the buffer using 0.22 μm filter. Store the 10x buffer at 4 °C as the solution stock3.
NOTE: The 10x Ringer’s buffer should be used within 3 months. - On the day of the experiment, make 1x goldfish Ringer’s buffer by diluting the 10x goldfish Ringer’s buffer using reverse osmosis water.
NOTE: The 1x goldfish Ringer’s buffer is used to saturate the PVA sponge, including the sponge used for the placement of larval zebrafish, and the sponge on the recording electrode tip.
2. Electrode Preparation
- Prepare the cone-shaped sponge recording electrode.
- Cut the male end from the platinum electrode lead extension and remove from the end 10 mm of the outer polytetrafluoroethylene insulation coating using a scalpel blade. Take care not to damage the inner wire of the electrode lead.
- Cut a 40 mm length of silver wire (0.3 mm diameter) and securely attach this to the electrode lead by entwining the silver wire with the exposed inner wire. Encase the joint using insulating tape, leaving a ~15 mm length of silver wire exposed (Figure 1A).
- Electroplate the exposed silver wire with chloride using a 9 V DC source for 60 s to improve signal conduction. Immerse the exposed silver tip in normal saline and connect the other end to the positive terminal of the battery. Connect another wire to the negative terminal of the battery and immerse the other end of the wire into the saline2.
NOTE: Alternatively, chlorinate the silver wire by soaking it for 1 hour in a bleach solution (active ingredient 42 g/L sodium hypochlorite). - Cut a ~20 mm x 20 mm square of PVA sponge using scissors to make a cone (Figure 1A). Saturate the sponge using 1x Ringer’s buffer. Under a microscope with a scale bar on the eyepiece, use a scalpel blade to shape the apex of the cone to ~40 µm diameter. Air dry the cone-shaped sponge on absorbent paper tissue until it is solid.
NOTE: The PVA sponge expands significantly when saturated, thus it is important that the sponge is first saturated with saline before shaping the apex of the cone. - After chloriding, air dry the silver wire on an absorbent tissue for 5 min. Insert the silver wire into the dried, solid, cone-shaped PVA sponge through the base of the cone. Insulate any excess exposed metal using mask tape to reduce photovoltaic artifacts (Figure 1B-C).
NOTE: After each experimental session, remove the sponge from the silver wire. Wash the sponge using reverse osmosis water and air dry for reuse. To ensure optimal signal collection, single use of silver wire is recommended. PVA sponges should not be reused more than 5 times. - On the day of the experiment, immerse the sponge-tip of the recording electrode into 1x Ringer’s buffer for at least 15 min to fully saturate the sponge.
- Prepare reference electrodes as described above, but without attaching the sponge tip.
- Obtain the ground electrode commercially.
3. Zebrafish Preparation
- Dark adapt zebrafish larvae overnight (>8 h) prior to recordings by placing zebrafish in a 15 mL tube (<20 larvae per tube) wrapped in aluminum foil in a dark incubator. Remove the lid to ensure adequate oxygen supply.
- On the day of recording, tighten the lid to the foil-wrapped falcon tube containing larvae and ensure that the tube is light-proof. Transport larvae to the ERG lab.
- Pour the fish into Petri dishes in the dark with the assistance of dim red illumination from a light-emitting diode (LED; 17.4 cd.m-2, λmax 600 nm). Cover Petri dishes using light-proof towels to minimize light exposure.
4. Sponge Platform Preparation
- Cut a rectangle of dry PVA sponge to fit snugly in a 35 mm Petri dish. Ensure that the thickness of the sponge should be roughly equal to the depth of the petri dish.
- Make a small cut vertically through one end of the sponge to accommodate the silver wire of the reference electrode.
- Soak the PVA sponge in 1x goldfish Ringer’s buffer until saturated. Then, place the sponge in a clean 35-mm petri dish. Use a paper towel to absorb extra liquid until no solution exudes from the sponge in response to a light finger press.
5. Animal and Electrode Positioning
- Anesthetize the larvae using 0.02 % tricaine diluted in 1x goldfish Ringer’s buffer.
- Use a 3 mL Pasteur pipette to transfer an anesthetized larva onto a square of paper towel (~3 cm2).
- Place the paper towel containing the larva on the moist sponge platform using forceps. Use a fine brush soaked in Ringer’s buffer to adjust the position of the larva. Ensure that one eye faces upwards, isolated from any nearby liquid on the square of paper towel underneath the larva.
- Glaze the larval body, excluding the head, with moisturizing eye gel to keep the larva moist throughout the ERG recording.
- Position the Petri dish with sponge platform on a small water-heated platform in front of the Ganzfeld bowl light stimulus situated inside a Faraday cage (Figure 1D).
NOTE: Maintenance of the temperature of the sponge and the larval body ensures stable ERG signals. - Insert the reference electrode into the cut made in the platform sponge (Figure 1D).
- Connect the commercially obtained ground electrode to the Faraday cage.
- Attach the recording electrode to an electrode holder and secure the holder to the stereotaxic arm of a micromanipulator (Figure 1D). Use a 3 mL Pasteur pipette to drip one drop of 1x Ringer’s solution on the sponge tip of the electrode for re-saturation.
- Position the microscope in the Faraday cage over the ERG platform for placement of the electrode.
NOTE: Illumination should be provided by a dim red LED (17.4 cd.m-2, λmax 600 nm) to allow observation of the larva and placement of the active electrode, whilst maintaining dark-adaptation. - Adjust the position of the sponge platform to allow observation of the larva under the microscope. Then, use absorbent tissue to remove excess liquid from the electrode sponge tip.
- Position the active electrode so that it gently touches the central corneal surface of the larval zebrafish eye (Figure 1E).
- Move the Ganzfeld bowl towards the sponge platform and ensure that the larva is covered by the bowl.
- Close the Faraday cage to reduce extraneous electromagnetic noise.
6. Electroretinogram Recording
- Use the computer software of the particular ERG system (see Table of Materials for details) to trigger the stimulus and acquire data based on the settings recommended below2.
- Set the sampling rate of the system to 4 KHz over a 650 ms recording window (2,560 points) in the acquisition software.
- Set the gain of the system to 1,000×.
- Set band-pass filtering of the system to 1–300 Hz.
- Use a notch filter to reduce 60 Hz (or 50 Hz, depending on local utility frequency) noise.
NOTE: The ideal noise level should be no more than ±10 µV.
- Commence data collection using the procedure described below.
- Use a single test-flash (0.06 log cd.s/m2) to measure a test response from the eye to assess the positioning of electrodes.
NOTE: This intensity of test flash should result in a b-wave amplitude greater than 25 µV in 4-dpf larvae. If a robust response cannot be measured, then reposition the electrodes and do another test flash to confirm that electrodes are well positioned. - Following the test-flash, allow the animal to dark adapt for 3 min in complete darkness before recordings.
- Present flashes from dimmer to brighter light intensities.
- Average signals across repeats according to the signal-to-noise level.
NOTE: Generally, average more signals at the dimmer light levels (no fewer than 3 repeats) and fewer at the brighter light levels (usually 1 repeat). Gradually lengthen the inter-stimulus interval from 10 to 60 s from the dimmest to brightest light level. A sample protocol is shown in Table 1. - After the recordings, humanely kill larvae using 0.1% tricaine.
- Use a single test-flash (0.06 log cd.s/m2) to measure a test response from the eye to assess the positioning of electrodes.
7. Analysis
- Measure the a-wave amplitude from baseline to the negative a-wave trough and the b-wave amplitude from the negative a-wave trough to the positive b-wave peak.
- Measure the a- and b-wave implicit times from stimulus onset to the trough of the a-wave and the peak of the b-wave, respectively.
Representative Results
This section provides representative results for ERG measurements taken daily from 4 to 7 dpf. From 4 dpf, ERG responses show robust a- and b-wave components, which arise from photoreceptors and bipolar cells, respectively. At each age tested, the amplitude of the b-wave increased with light intensity (Figure 2; Figure 3). Notably, the sensitivity of the larval zebrafish retina to dimmer flashes increased with age. The a- and b-wave were not recognizable at intensities lower than -1.61 log cd.s/m2 at 4 dpf, whereas clear signals were detectable at these intensities for older larvae (Figure 2). The b-wave response grew substantially between 4 and 5 dpf (P < 0.0001; Figure 2A-B; Figure 3B). Although the b-wave at lower intensities showed little change between 5 and 7 dpf, the signal at 2.48 log cd.s/m2 was greater at 7 dpf compared with 5 and 6 dpf (P < 0.0001; Figure 2; Figure 3B). A- and b-wave implicit times became significantly faster after 5 dpf (P < 0.0001; Figure 3C-D). Overall, these results demonstrate maturation of zebrafish retinal function between 4 to 7 dpf. Interestingly, the a-wave amplitude appeared to decrease from 5 to 7 dpf (Figure 3A). This may be because the maturation of synaptic connections in the outer retina shortens the latency of bipolar cells responses, resulting in faster b-wave onset that masks the a-wave. Those wishing to study the a-wave can employ pharmacological treatment to block post-photoreceptoral responses (i.e. the b-wave component).
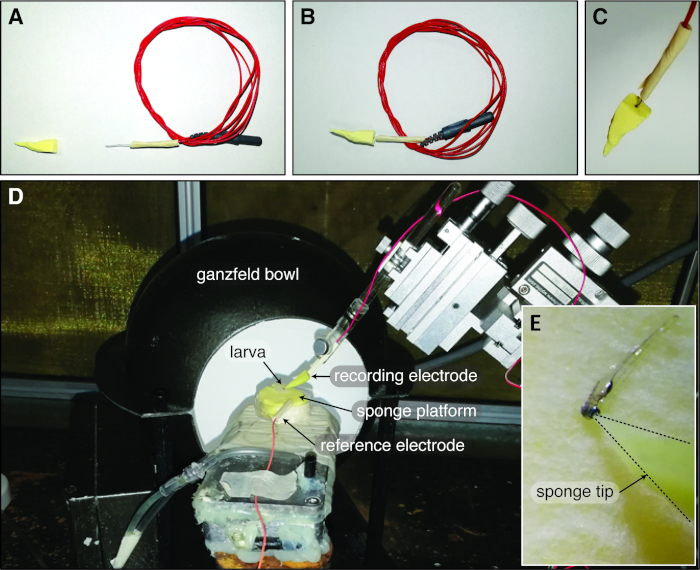
Figure 1: Zebrafish Ganzfeld ERG set up with the cone-shaped sponge-tip electrode. (A) The cone-shaped sponge tip and the chlorinated silver electrode are air dried before constructing the sponge-tip electrode. (B-C) Subsequently, the chlorinated silver wire is inserted into the sponge cone through the base to form the complete electrode. (D) In the typical larval zebrafish Ganzfeld ERG setup, the reference electrode is inserted into the sponge platform and the zebrafish larva is covered by the Ganzfeld bowl. (E) The sponge-tip electrode gently touches the central corneal surface of the larval eye. Please click here to view a larger version of this figure.
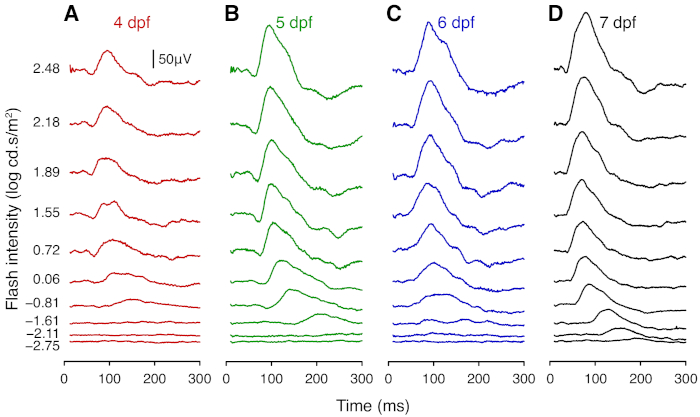
Figure 2: Representative average ERG traces of wild-type larval zebrafish. Average ERG traces of wildtype zebrafish at (A) 4 dpf (n = 8), (B) 5 dpf (n = 8), (C) 6 dpf (n = 7), and (D) 7 dpf (n = 9). Responses were elicited using flashes from white LEDs. At each age, the traces show responses to flashes of (from bottom to top) -2.75, -2.11, -1.61, -0.81, 0.06, 0.72, 1.55, 1.89, 2.18, 2.48 log cd.s/m2. Scale bar = 50 µV. Please click here to view a larger version of this figure.
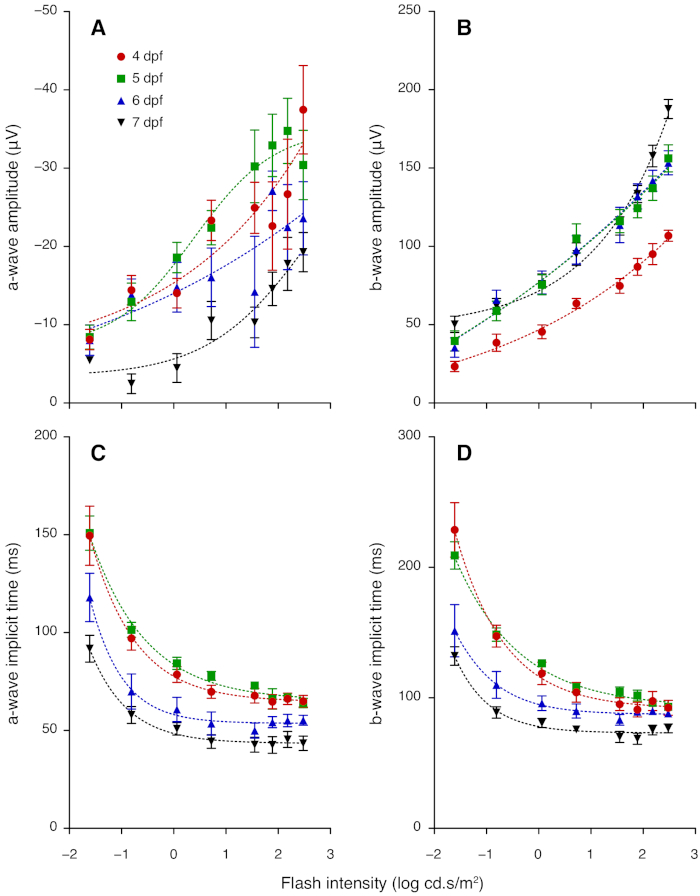
Figure 3: ERG a- and b-wave amplitudes and implicit times for 4 to 7 dpf zebrafish. (A) Group average (± standard error of the mean) a-wave amplitude increased with flash intensity but decreased with age in 4–7 dpf larvae. (B) Average b-wave amplitude in 4–7 dpf larvae increased with flash intensity; amplitude grew between 4 and 5 dpf. (C) Average a-wave implicit time and (D) average b-wave implicit time became faster between 5, 6 and 7 dpf. Lines of best fit are derived from non-linear regression. Please click here to view a larger version of this figure.
| Stimulus light intensity (log cd.s.m-2) | Number of repeats | Inter-stimulus interval (s) |
| -2.75 | 3 to 6 | 10 |
| (30 s before next) | ||
| -2.11 | 3 to 6 | 10 |
| (30 s before next) | ||
| -1.61 | 3 to 6 | 10 |
| (30 s before next) | ||
| -0.81 | 3 to 6 | 10 |
| (60 s before next) | ||
| 0.06 | 3 to 6 | 10 |
| (60 s before next) | ||
| 0.72 | 1 to 3 | 60 |
| 1.55 | 1 to 3 | 60 |
| 1.89 | 1 to 3 | 60 |
| 2.18 | 1 to 3 | 60 |
| 2.48 | 1 to 3 | 60 |
Table 1: Example protocol of ERG recordings. Stimulus presentations start from the dimmest (top) and progress to brighter (bottom) light levels, with progressively longer inter-stimulus intervals to ensure that dark adaption is maintained. The number of signals averaged at each intensity depends on the signal-to-noise level.
Discussion
Functional readouts such as the ERG have become increasingly important in the suite of tools used to study larval zebrafish8,9,12,14. Due to the small size of the larval zebrafish eye, glass micropipettes have been adapted as recording electrodes in most published protocols3,4,5,8,9,12,13,14. Here we describe a larval zebrafish ERG protocol using a simpler cone-shaped sponge-tip electrode. The novel electrode can be used to modify standard small-animal ERG systems to measure larval zebrafish retinal function without any additional equipment. The materials for making the sponge-tip electrode are simply commercial PVA sponge and 0.3 mm silver wire, which makes this more economical than previous approaches. Another advantage is that, in contrast to the hard and sharp micropipette tip, the gentler electrode sponge tip is less likely to damage the larval eye. Finally, the PVA sponge helps to maintain moisture to the larval eye throughout the recording.
The key to successful application of the sponge-tip electrode is to ensure full saturation of the sponge. This normally takes no less than 15 minutes of soaking in 1x goldfish Ringer’s buffer. Incomplete saturation of the sponge can increase the noise level owing to faster drying of the electrode. For better signal collection, making new electrodes for each experimental session (generally < 8 h) is highly recommended. Repeat use can lead to reduced ERG signals, making inter-session comparisons more difficult.
When positioning the larval zebrafish onto the sponge platform, care must be taken to ensure that the eye to be measured is not in contact with any surrounding solution or the paper towel underneath the fish. Such contact shorts the electrical circuit, as the reference electrode is embedded in the sponge platform and reduces the ERG.
Even with well saturated electrode sponge tips, gradual drying occurs, which is evident as increased noise in ERG signals. Should this occur, drip one drop of 1x goldfish Ringer’s onto the base of the cone using 1 mL syringe and a 30 G x ½" needle. If adding the solution to the sponge tip does not reduce the noise level, check that the eye is not in contact with a surrounding fluid and ensure that the electrode tip is centered on the corneal apex.
The recordings in the representative results reported here were made with a bandpass setting of 1–300 Hz, which does not allow sampling of oscillatory potentials (OP)—wavelets on the b-wave derived from the third-order retinal neurons including amacrine and ganglion cells 15,16,17. A higher lowpass setting (e.g., 500 or 1,000 Hz) may be better suited for OP recording.
In summary, the cone-shaped sponge-tip electrode helps to simplify larval zebrafish ERG recording with existing small-animal ERG systems, providing reliable results. Representative results demonstrate that ERG amplitude grows between 4 and 5 dpf, with further maturation between 5 and 7 dpf manifesting as faster implicit times. Our simple ERG protocol with the economical and practical cone-shaped sponge-tip electrode can benefit investigators studying zebrafish retinal function. The technique can also be adapted to assess adult zebrafish or other vertebrate models with small eyes.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
Funding for this project was provided by a grant from the Melbourne Neuroscience Institute (to PTG, PRJ & BVB).
Materials
| 0.22 µm filter | Millex GP | SLGP033RS | Filters the 10× goldfish ringer's buffer for sterilizatio |
| 1-mL syringe | Terumo | DVR-5175 | With a 30G × ½" needle to add drops of saline to the electrode sponge tip to prevent drying and increased noisein the ERG signals. |
| 30G × ½" needle | Terumo | NN*3013R | For adding saline toteh sopnge tip electrode. |
| Bioamplifier | ADInstruments | ML135 | For amplifying ERG signals. |
| Bleach solution | King White | 9333441000973 | For an alternative method of sliver electrode chlorination. Active ingredient: 42 g/L sodium hypochlorite. |
| Circulation water bath | Lauda-Königshoffen | MGW Lauda | Used to make the water-heated platfrom. |
| Electrode lead | Grass Telefactor | F-E2-30 | Platinum cables for connecting silver wire electrodes to the amplifier. |
| Faraday Cage | Photometric Solution International | For maintianing dark adaptation and enclosing the Ganzfeld setup to improve signal-to-noise ratio. | |
| Ganzfeld Bowl | Photometric Solution International | Custom designed light stimulator: 36 mm diameter, 13 cm aperture size. | |
| Luxeon LEDs | Phillips Light Co. | For light stimulation twenty 5W and one 1W LEDs. | |
| Micromanipulator | Harvard Apparatus | BS4 50-2625 | Holds the recording electrode during experiments. |
| Microsoft Office Excel | Microsoft | version 2010 | Spreadsheet software for data analysis. |
| Moisturizing eye gel | GenTeal Gel | 9319099315560 | Used to cover zebrafish larvae during recordings to avoiding dehydration. Active ingredient: 0.3 % Hypromellose and 0.22 % carbomer 980. |
| Pasteur pipette | Copan | 200C | Used to caredully transfer larval zebrafish. |
| Powerlab data acquisition system | ADInstruments | ML785 | Controls the LEDs to generate stimuli. |
| PVA sponge | MeiCheLe | R-1675 | For the placement of larval zebrafish and making the cone-shaped electrode ti |
| Saline solution | Aaxis Pacific | 13317002 | For electroplating silver wire electrode. |
| Scope Software | ADInstruments | version 3.7.6 | Simultaneously triggers the stimulus through the Powerlab system and collects data |
| Silver (fine round wire) | A&E metal | 0.3 mm | Used to make recording and reference ERG electrodes. |
| Stereo microscope | Leica | M80 | Used to shape and measure the cone-shaped sponge apex (with scale bar on eyepiece). Positioned in the Faraday cage for electrode placement. |
| Tricaine | Sigma-aldrich | E10521-50G | For anaethetizing larval zebrafish. |
| Water-heated platform | custom-made | For maintianing the temperature of the sponge platform and the larval body during ERG recordings |
Referenzen
- Roper, C., Tanguay, R. L., Slikker, W., Paule, M. G., Wang, C. . Handbook of Developmental Neurotoxicology (Second Edition). , 143-151 (2018).
- Nguyen, C. T., et al. Simultaneous Recording of Electroretinography and Visual Evoked Potentials in Anesthetized Rats. Journal of visualized experiments: JoVE. , e54158 (2016).
- Chrispell, J. D., Rebrik, T. I., Weiss, E. R. Electroretinogram analysis of the visual response in zebrafish larvae. Journal of visualized expriment: JoVE. (97), (2015).
- Seeliger, M. W., Rilk, A., Neuhauss, S. C. Ganzfeld ERG in zebrafish larvae. Documenta Ophthalmologica. 104 (1), 57-68 (2002).
- Fleisch, V. C., Jametti, T., Neuhauss, S. C. Electroretinogram (ERG) Measurements in Larval Zebrafish. Cold Spring Harbor Protocols. 2008, (2008).
- Biehlmaier, O., Neuhauss, S. C., Kohler, K. Synaptic plasticity and functionality at the cone terminal of the developing zebrafish retina. Developmental Neurobiololgy. 56 (3), 222-236 (2003).
- Gestri, G., Link, B. A., Neuhauss, S. C. The visual system of zebrafish and its use to model human ocular diseases. Developmental Neurobiololgy. 72 (3), 302-327 (2012).
- Saszik, S., Bilotta, J., Givin, C. M. ERG assessment of zebrafish retinal development. Visual Neuroscience. 16 (5), 881-888 (1999).
- Niklaus, S., et al. Cocaine accumulation in zebrafish eyes leads to augmented amplitudes in the electroretinogram. Matters. 3 (6), e201703000003 (2017).
- Tanvir, Z., Nelson, R. F., DeCicco-Skinner, K., Connaughton, V. P. One month of hyperglycemia alters spectral responses of the zebrafish photopic ERG. Disease models & mechanisms. , (2018).
- Kakiuchi, D., et al. Oscillatory potentials in electroretinogram as an early marker of visual abnormalities in vitamin A deficiency. Molecular medicine reports. 11 (2), 995-1003 (2015).
- Emran, F., Rihel, J., Adolph, A. R., Dowling, J. E. Zebrafish larvae lose vision at night. Proceedings of the National Academy of Sciences. , (2010).
- Makhankov, Y. V., Rinner, O., Neuhauss, S. C. An inexpensive device for non-invasive electroretinography in small aquatic vertebrates. Journal of Neuroscience Methods. 135 (1-2), 205-210 (2004).
- Bilotta, J., Saszik, S., Sutherland, S. E. Rod contributions to the electroretinogram of the dark-adapted developing zebrafish. Developmental Dynamics. 222 (4), 564-570 (2001).
- Cameron, M. A., Barnard, A. R., Lucas, R. J. The electroretinogram as a method for studying circadian rhythms in the mammalian retina. Journal of genetics. 87 (5), 459-466 (2008).
- Bui, B. V., Armitage, J. A., Vingrys, A. J. Extraction and modelling of oscillatory potentials. Documenta Ophthalmologica. 104 (1), 17-36 (2002).
- Bui, B. V., Fortune, B. Ganglion cell contributions to the rat full-field electroretinogram. The Journal of Physiology. 555 (1), 153-173 (2004).

