Repeated Blood Collection from Tail Vein of Non-Anesthetized Rats with a Vacuum Blood Collection System
Summary
Here, we describe a simple tail vein blood sampling method in non-anesthetized rats using a vacuum extraction tube system. This method reduces the risk of direct exposure to blood and simplifies taking multiple samples from a single venipuncture.
Abstract
Blood can be collected from rats in a number of sampling locations. For instance, the tail vein is a superior location for blood sampling. However, the tail vein is thin so that it is sometimes hard to puncture. In addition, the tail vein has low blood flow and requires a long sampling time to get sufficient blood. The present report describes a simple blood sampling method, the vacuum blood collection method, which is usually used to obtain blood samples from patients, here used for non-anesthetized rats. The 22 G butterfly needle tip was inserted into one of the lateral tail veins approximately 2-3 cm from the tip of the tail at an angle of approximately 20°, and blood was collected in the vacuum collection tube by inserting the rubber end of the butterfly needle into the vacuum blood collection tube. The present experimental results show that the success rate was 95% in the experimental group and 90% in the beginner group. The success rate and puncture times were similar between two groups. The sampling duration was significantly shorter in the experimental group compared to beginner group. In conclusion, this vacuum blood collection method for sequential blood sampling from the tail vein of non- anesthetized rats is feasible and easy-to-learn, which might serve as a reliable alternative to other conventionally used blood sampling techniques for rats.
Introduction
Blood sampling from rats is required for a wide variety of experimental studies. Techniques for blood collection from the rat include puncture of the heart, retro-orbital plexus, jugular vein, saphenous vein, tail blood vessels, carotid artery, abdominal aorta, and vena cava. Most techniques (except saphenous vein and tail blood vessel puncture) require anesthesia1,2. Veins of the rat tail are routinely punctured for blood sampling and vascular injection. After treating the tail with warm water, the blood vessels are well dilated and suitable for manipulation, even when rats are anesthetized3.
Despite the development of new and improved techniques to obtain blood samples in small laboratory animals2, it is not always easy to obtain enough blood when sampling from small animals. The tail vein of rats is a superior location for blood sampling4. However, the tail vein is thin, thus it is sometimes hard to puncture and would take a long time to get enough blood due to its low blood flow. Blood collection via a vacuum extraction tube system is a typical method in daily clinical practice. This closed system for blood collecting reduces the risk of direct exposure to blood and has made it easier to take multiple samples from a single venipuncture5. The present report describes a simple tail vein blood sampling method in non-anesthetized rats using the vacuum extraction tube system. This method is easy to master and can be used to obtain a large volume of blood repeatedly in rats.
Protocol
All procedures were approved by the Tongji Medical College Council on the Animal Care Committee of Huazhong University of Science and Technology (Wuhan, China). The manuscript was prepared according to ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines6.
1. Husbandry
- Use Sprague-Dawley (SD) rats (n = 20, 10 males, 8 week-old, weighing 261 to 291 g, mean = 272.85 ±9.07 g) from the Experimental Animals Center of Huazhong University of Science and Technology and maintain rats in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No.85-23, revised 1996)
- House under standard conditions with free access to food and drinking water. Keep rats in 530 cm2 cages with wood-shaving bedding. House two rats in each cage. Keep all cages open to the room environment (no micro-isolation or ventilated caging)
- Ensure the staff involved in the experimental protocol regularly handle the rats.
- Maintain room temperature between 21 °C and 23 °C. Give rates a normal salt diet (0.3% NaCl) throughout the study.
2. Blood Collection Procedure
NOTE: The vacuum blood collection system consists of a double-pointed needle [22 G (0.7 mm x 25 mm)], a plastic holder, and a series of vacuum tubes (2 mL) with rubber stoppers (Figure 1).
- Place the rat in a plastic restraining holder and wash the tail with warm (20-30 °C) water to remove any visible dirt or feces. While an assistant holds the tail in an extended position, wipe the tail with 70% ethanol, and clean with gauze to make the vein clear.
- Insert the 22 G butterfly needle tip into one of the lateral tail veins (around 5 mm) at a position approximately 2-3 cm away from the tip of the tail at angle of approximately 20° (Figure 2). Collect blood into the vacuum collection tube by inserting the rubber end of the butterfly needle into the vacuum blood collection tube (Figure 3).
NOTE: The maximal collection blood volume is 1.2 mL7. - After blood collection, remove the needle and gently apply pressure to the puncture site with gauze for 15-30 s to stop the flow of blood. Then, release the rat from the plastic restraining holder and return the rat to its cage.
- Use tubes that contain EDTA as an anticoagulant to collect blood plasma. Gently invert the tube several times to mix anticoagulant in the blood and place the samples on ice vertically.
- Centrifuge the blood sample collection tubes in a refrigerated centrifuge at 2,000 x g for 10 min to separate plasma or serum.
- Extract the plasma/serum, taking care not to disturb the red and white blood cell layers. To collect blood serum, use tubes without anticoagulant. Both types of sample can be used immediately, or stored at -80 °C for up to one year.
NOTE: Successful blood collection was defined as obtaining 1.2 mL blood each time. Maximal puncture time was set to three trials, if the total blood volume was lower than 1.2 mL after the third puncture, it was defined as a failed blood collection. The sampling duration starts from the tail vein puncture to the removal of the butterfly needle after blood collection. - Collect blood twice within two weeks7.
- To test and verify the feasibility of this method, train two medical students (beginners) for 2 h before performing this blood collection protocol. Use twenty rats and draw blood from 10 rats by 2 experienced fellows (experimental group) and from 10 rats by the 2 medical students (beginner group).
3. Statistical Analysis
- Express data as a mean value ± standard deviation and analyze with SPSS Statistics 17. An α value of 0.05 was chosen, therefore p <0.05 was considered statistically significant.
Representative Results
Sampling duration, body mass, and blood collection volume of the two groups
Blood was collected from 10 rats (5 males) twice within two weeks in each group. The mean body mass in both groups was similar (272.00 ±9.66 g and 273.70 ±8.87 g). Sampling duration was significantly shorter in the experimental group compared to beginner group (2.77 ±0.53 min vs. 3.28 ±0.83 min, p <0.05) (Table 1).
Success rate and puncture times of two groups
The success rate was 95% (19/20) in the experimental group and 90% (18/20) in the beginner group (p >0.05). Puncture times were not significantly different between the two groups (1.50 ±0.61 min vs. 1.85 ±0.75 min, p >0.05) (Table 2). These results indicate this method is easy to learn, and the technique could be mastered after training for 2 h.
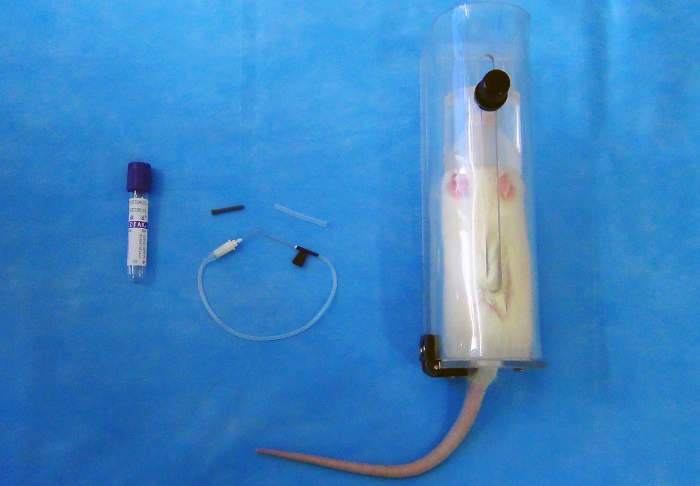
Figure 1: 2 mL vacuum blood collection tube (left); 22 G butterfly needle (center); the plastic restraining holder (right). Please click here to view a larger version of this figure.

Figure 2: The needle tip was inserted into the lateral tail vein (around 5 mm) at the position approximately 2-3 cm away from the tip of the tail at angle of approximate 20°. Please click here to view a larger version of this figure.
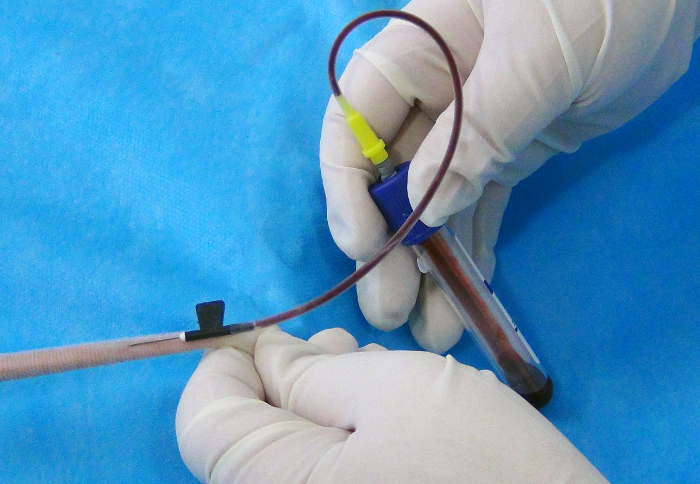
Figure 3: Successful venipuncture. Please click here to view a larger version of this figure.
| Experimental group | Beginner group | |||||||||
| Gender | Body weight (g) | Collected blood volume (mL) | sampling duration (min) | Gender | Body weight (g) | Collected blood volume (mL) | sampling duration (min) | |||
| E1-1 | male | 291 | 1.2 | 3.12 | B1-1 | male | 282 | 1.2 | 3.18 | |
| E1-2 | male | 291 | 1.2 | 2.73 | B1-2 | male | 282 | 1.2 | 2.18 | |
| E2-1 | male | 276 | 1.2 | 2.3 | B2-1 | male | 264 | 1.2 | 3.73 | |
| E2-2 | male | 276 | 1.2 | 2.37 | B2-2 | male | 264 | 1.2 | 2.47 | |
| E3-1 | male | 267 | 1.2 | 2.73 | B3-1 | male | 265 | 1.2 | 3.52 | |
| E3-2 | male | 267 | 1.0 | 3.88 | B3-2 | male | 265 | 1.2 | 2.53 | |
| E4-1 | male | 263 | 1.2 | 2.41 | B4-1 | male | 289 | 1.2 | 2.32 | |
| E4-2 | male | 263 | 1.2 | 2.52 | B4-2 | male | 289 | 1.2 | 3.62 | |
| E5-1 | male | 265 | 1.2 | 3.61 | B5-1 | male | 277 | 1.2 | 3.48 | |
| E5-2 | male | 265 | 1.2 | 2.42 | B5-2 | male | 277 | 1.2 | 3.12 | |
| E6-1 | female | 285 | 1.2 | 3.33 | B6-1 | female | 274 | 0.9 | 4.87 | |
| E6-2 | female | 285 | 1.2 | 3.03 | B6-2 | female | 274 | 1.2 | 3.37 | |
| E7-1 | female | 271 | 1.2 | 2.82 | B7-1 | female | 268 | 1.0 | 3.93 | |
| E7-2 | female | 271 | 1.2 | 2.42 | B7-2 | female | 268 | 1.2 | 3.82 | |
| E8-1 | female | 268 | 1.2 | 1.93 | B8-1 | female | 262 | 1.2 | 2.07 | |
| E8-2 | female | 268 | 1.2 | 3.05 | B8-2 | female | 262 | 1.2 | 3.67 | |
| E9-1 | female | 273 | 1.2 | 2.52 | B9-1 | female | 281 | 1.2 | 4.38 | |
| E9-2 | female | 273 | 1.2 | 3.17 | B9-2 | female | 281 | 1.2 | 4.57 | |
| E10-1 | female | 261 | 1.2 | 1.82 | B10-1 | female | 275 | 1.2 | 2.53 | |
| E10-2 | female | 261 | 1.2 | 3.25 | B10-2 | female | 275 | 1.2 | 2.28 | |
| Average | 2.77 | 3.28 | ||||||||
| En-1: serial number of rats in the experimental group for the first blood collection. | ||||||||||
| En-2: serial number of rats in the experimental group for the second blood collection. | ||||||||||
| Bn-1: serial number of rats in the beginner group for the first blood collection. | ||||||||||
| Bn-2: serial number of rats in the beginner group for the second blood collection. | ||||||||||
Table 1: Results of blood collected in the experimental group and the beginner group.
| Blood collection times | Average puncture times | One time puncture | Two times puncture | Three times puncture | Failed | Success rate | |
| Experimental group | 20 | 1.50 ±0.61 | 11 | 8 | 0 | 1 | 95% |
| Beginner group | 20 | 1.85 ±0.75 | 7 | 9 | 2 | 2 | 90% |
| One time puncture: Number of rats with successful blood collection after the first puncture | |||||||
| Two times puncture:Number of rats with successful blood collection after the second puncture | |||||||
| Three times puncture:Number of rats with successful blood collection after the third puncture | |||||||
| Failed:Number of rats with failed blood collection after the third puncture | |||||||
Table 2: Comparison of the puncture times and success rates of the experimental group and the beginner group.
Discussion
The present report describes an easy-to-learn blood collection method in rats. Our results showed that this method is feasible for repeatedly collecting a large volume of blood from rats. Moreover, this method could be easily mastered with a short-term learning curve. This procedure can be performed in non-anesthetized rats and only causes minimal stress to the animals.
Blood can be collected from rats in various locations. The tail vein is a superior location for blood sampling4. However, it is not always easy to obtain enough blood volume from the thin and low blood flow tail vein of the rats. The vacuum blood collection system is usually used for collecting blood samples from patients in daily clinical practice. This vacuum blood collection system has been used in patients and research animals for a long time8,9,10. This system consists of a vacuum blood collection tube with silicone coated inner tube wall and butterfly needle. It was based on the vacuum negative pressure principle and allows the continuous drawing of blood without the risk of blood collecting, which also reduces the chance of contamination and hemolysis11. The volume of blood sample in the catheter is about 0.3 mL. After withdrawing the needle from the tail vein, the blood blocked in the catheter will flow into the collecting vacuum tube due to the vacuum. This method is also applicable for collecting blood from rats weighing less than 100 g with the use of a smaller puncture needle.
This blood collection method has several advantages. This procedure only causes minimal injury to rats and blood collection can be performed without the use of anesthetics, thus the influence of the stress response and anesthetics on the blood sample can be avoided. Our observations showed that rats were quiet in the plastic restraining holder during the procedure and there were no deaths during these experiments. Secondly, repeated blood collection every one or two days is possible, which enables frequent sampling of blood with an adequate volume for various research purposes. Finally, this method is easy to learn and was easily mastered by beginners after a short (2 h) learning period, as indicated by the similar success rates and puncture times between the experimental and beginner groups in this report. In a previous report, Lee et al. demonstrated a similar method of sampling blood from the lateral tail vein of the rat4. Our method is simpler than that reported by Lee et al.4 in that we directly insert the puncture needle into the tail vein and the other end of the tube is directly connected to the vacuum tube for collecting the blood, without the use of an extra syringe and manual aspiration of the blood and without the need of 'milking' the vein to facilitate blood flow. Collecting blood with this closed system reduces the chance of blood contamination. Use of the plastic restraining holder also reduces the stress to the rat and facilitates the fixation of the tail.
The following two steps are critical for the successful application of this method. 1) Extend the tail and avoid the movement of the tail; 2) Gently puncture the vein at an angle of 20° to avoid puncture through the vein and keep the puncture needle in the vein.
In conclusion, the adopted vacuum blood collection method in rats is safe, feasible, and easy to practice. This method enables frequent sampling of blood with an adequate blood volume obtained from non-anesthetized rats.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements to make.
Materials
| a double-pointed needle | Shanghai Kang Nong medical instrument co., LTD, China | 22G | (0.7×25mm) |
| vacuum tubes | Wuhan Zhi Yuan, medical science and technology co., LTD, China | 2 ml | |
| rat restraining holder | Shanghai Kang Nong medical instrument co., LTD, China | 250g rat hoder model | |
| normal salt diet for rats | Rats received a normal salt diet (0.3% NaCl) throughout the study | ||
| SPSS software for statistical analysis | SPSS Inc, Chicago; USA | Version 17.0. |
Referenzen
- Brown, C. Blood collection from the tail of a rat. Lab Anim (NY). 35 (8), 24-25 (2006).
- Parasuraman, S., Raveendran, R., Kesavan, R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 1 (2), 87-93 (2010).
- Staszyk, C., Bohnet, W., Gasse, H., Hackbarth, H. Blood vessels of the rat tail: a histological re-examination with respect to blood vessel puncture methods. Lab Anim. 37 (2), 121-125 (2003).
- Lee, G., Goosens, K. A. Sampling blood from the lateral tail vein of the rat. J. Vis. Exp. (99), (2015).
- . . WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy WHO Guidelines Approved by the Guidelines Review Committee. , (2010).
- Kilkenny, C., Altman, D. G. Improving bioscience research reporting: ARRIVE-ing at a solution. Lab Anim. 44 (4), 377-378 (2010).
- Diehl, K. H., et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 21 (1), 15-23 (2001).
- Eder, J. M., Cutter, G. R. A new device for collecting cord blood. Obstet Gynecol. 86 (5), 850-852 (1995).
- Reynolds, B. S., et al. Comparison of a new device for blood sampling in cats with a vacuum tube collection system – plasma biochemistry, haematology and practical usage assessment. J Feline Med Surg. 9 (5), 382-386 (2007).
- Wiwanitkit, V. Comparison of blood specimens from plain and gel vacuum blood collection tubes. J Med Assoc Thai. 84 (5), 723-726 (2001).
- Wollowitz, A., Bijur, P. E., Esses, D., John Gallagher, E. Use of butterfly needles to draw blood is independently associated with marked reduction in hemolysis compared to intravenous catheter. Acad Emerg Med. 20 (11), 1151-1155 (2013).

