Tuning a Parallel Segmented Flow Column and Enabling Multiplexed Detection
Summary
Here, we present a protocol for the operation and tuning of parallel segmented flow chromatography columns to enable multiplexed detection.
Abstract
Active flow technology (AFT) is new form of column technology that was designed to overcome flow heterogeneity to increase separation performance in terms of efficiency and sensitivity and to enable multiplexed detection. This form of AFT uses a parallel segmented flow (PSF) column. A PSF column outlet end-fitting consists of 2 or 4 ports, which can be multiplexed to connect up to 4 detectors. The PSF column not only allows a platform for multiplexed detection but also the combination of both destructive and non-destructive detectors, without additional dead volume tubing, simultaneously. The amount of flow through each port can also be adjusted through pressure management to suit the requirements of a specific detector(s). To achieve multiplexed detection using a PSF column there are a number of parameters which can be controlled to ensure optimal separation performance and quality of results; that is tube dimensions for each port, choice of port for each type of detector and flow adjustment. This protocol is intended to show how to use and tune a PSF column functioning in a multiplexed mode of detection.
Introduction
Active Flow Technology Columns
Active flow technology (AFT) chromatography columns were recently developed to overcome inefficiency in separations associated with flow heterogeneity1-6 and also to enable multiplexed detection. In this particular communication we detail the operational process of the parallel segmented flow (PSF) column with multiplexed detection. The key functional advantages of the PSF column are: (1) the flow from the radial central region of the column bed is isolated from the peripheral or wall region of flow, (2) the volume of mobile phase that must be processed by a detection source is reduced, and (3) detection sources can be multiplexed to expand sample information without instilling detection delays across each detection process, or subsequently necessitating the splitting of a post column flow stream7,8. The key feature in the design of the PSF column that enables the advantage of multiplexed detection is the novel outlet fitting and frit assembly. Figure 1 is a photograph of the AFT column compared to a conventional column. It is important to understand that the splitting process obtained using parallel segmented flow columns is not the same as post column flow stream splitting. In a post column flow stream split the entire sample band from the leading edge to the extremities of the tail is sampled equally (i.e., axially), thereby, each flow stream is equal with respect to efficiency and sensitivity; the magnitude of the sensitivity thereby being divided by the number splits. In PSF, however, the splitting process samples the band radially, not axially. As such, the central port samples the peak apex — the most concentrated region of the peak. Thus, the sensitivity here is highest as the peak is not diluted by the diffuse tailing region. The sample eluting from the peripheral ports is not as efficient as in the central zone, but, since the band is sampled radially, rather than axially, the width of the peak is narrower than would be the case for a sampling process that divides the peak in the axial direction, i.e., a post column split. Hence the sensitivity with a concentration dependent detector is not reduced.
In the PSF column, the outlet fitting comprises multiple exit ports and on the inside of this end fitting there is housed an annular frit. The inner portion of this annular frit channels flow out of the column via the radial central outlet port, while the outer radial portion of the outlet frit channels flow out of the column via the peripheral or wall region outlet flow ports. The inner and outer portions of the outlet frit are separated by an impermeable barrier that prevents cross flow between these flow regions2. As a consequence of this design the central radial flow stream through the column bed is separated from the wall region flow inside the column. The relative portion of flow from these two regions can be varied to almost any desired ratio through pressure management in order to optimize various functional aspects of the column technology, such as separation efficiency or detection sensitivity. In essence, this design effectively establishes within the larger format column a 'virtual' column, having a narrower internal diameter, and hence the column functions as a true wall-less column, overcoming column bed heterogeneity and the wall effects9,10.
The major benefits of PSF columns are improvements in column efficiency, minimization of the solvent processing for the detection source(s) and enabling multiplexed detection. However, an added advantage is that since the tailing and fronting regions of any band are removed from the overall elution profile the solute at elution or detection is present in a higher concentration than would otherwise be observed for the same solute injection and concentration load on a conventional column, depending on the segmentation ratio employed. As a consequence there is often observed a gain in signal intensity for separations conducted on PSF columns2. In fact, if the segmentation ratio is adjusted such that 25% of the flow exits from each of the four exit ports, the signal intensity that is observed using Ultra Violet (UV) detection shows practically the exact same signal intensity as apparent using a conventional column where the entire (100%) of the mobile phase is analyzed7. Furthermore, fine tuning of the outlet ratio between the central and wall flow regions allows the column efficiency to be optimized. The gains in column efficiency observed using AFT columns cannot be stated to a single value, since these efficiency gains are a function of three factors: (1) the flow rate, (2) the segmentation ratio, and (3) the solute retention factor. Nevertheless, gains in efficiency compared to conventional columns are almost always observed, and sometimes these gains are more than 100% in the number of theoretical plates1,2. The ability to tune the segmentation ratio allows the analyst to effectively tailor the diameter of the 'virtual' column, and this is an important factor in relation to the detection process. For example, a virtual 2.1 mm internal diameter (i.d.) column is established from a physical 4.6 mm i.d. column when the segmentation ratio is 21% of mobile phase eluting from the radial central exit port. Under these conditions, the virtual 2.1 mm i.d. column performs with an efficiency that may be more than 70% greater than the conventional 2.1 mm i.d. column, depending on flow rate, and solute retention factor10.
The current PSF column design that is used for multiplexed detection incorporates a 4-port outlet fitting, but the column can be fitted with a 2-port end-fitting also, however, this limits detection to only two detectors. The basic operation of these columns is, however, the same, except that four detectors can be coupled simultaneously to the 4-port outlet PSF column broadening the scope for multiplexed detection. Aside from pre- and post- column connective tubing, the only additional requirements to operate a PSF column is tubing that can be connected to the peripheral outlet ports, and a means by which the amount of mobile phase passing through each tube can be measured, typically either a mass measurement or a volumetric measurement. For ease of tuning, the internal diameter of all outlet flow tubing should be the same. The flow ratio between the peripheral and radial central exit ports is then varied through the use of pressure management, simply by changing the length of the tubing located on the peripheral outlet fitting, or the length of tubing post detector on the radial central outlet port.
Multiplexed Detection Using PSF Columns
An important advantage of PSF columns is that each of the outlet exit ports can be connected directly to a detection source, thus enabling multiplexed detection. In a well-designed detection system a single analysis with multiplexed detection can provide substantial information in regards to the nature of the components within the sample. Importantly, destructive and non-destructive tests can be conducted at exactly the same time, without detection delay. This allows the absolute assignment of, for example, anti-oxidants using DPPH• reagent, with components observed to elute with UV and/or mass spectrometry (MS) detection responses7,11. Therefore, four independent detectors can be operated simultaneously with appropriate portions of flow directed to each detector via any of the four outlet ports. Since the flow through these ports can be easily adjusted the amount of solute reaching any of the detectors can be adjusted to suit the sensitivity of the given detector source. It should be noted, however, that the most efficient solute migration is observed through the radial central outlet port. Each of the peripheral ports offer equivalent separation efficiency, which when set to 25% through each port, is only slightly less efficient than a conventional column. As such, it is important that the quantitative detector be set to analyze sample from the radial central exit port.
When setting up a PSF column for the purpose of multiplexing detection there are a number of considerations that need to be made to achieve efficient and high quality results; that is tube dimensions for each port, choice of which port for a type of detector and flow adjustment.
Tube Dimensions for Each Port
In chromatography the length of post column tubing plays a crucial role in the efficiency and performance of the separation. Large dead-volume as a result of long or wide i.d. tubing from column outlet to detector will result in a loss of efficiency, resolution and sensitivity. Thus, appropriate tubing dimensions must be utilized when setting up the PSF column to attain the maximum potential in providing efficient separation whilst providing the benefits of multiplexing.
Port to Detector
Figure 2 is an illustration of an example setup of multiplexed detection (Ultra Violet-Visible (U-Vis), mass spectrometer (MS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH•) detection). The illustration shows the central port is attached to the MS detector, whereas the DPPH• and UV-Vis detectors are attached to the peripheral ports. Since the MS is the most sensitive detector of the three, flow to this detector was directed from the central outlet port. As DPPH• detection is selective to the presence of antioxidants, and the least sensitive and most tolerant to band broadening, flow to this detector was directed from a peripheral port. The UV-Vis was a secondary 'generic' detector, so flow to this detector was directed from a second peripheral port.
Flow Adjustment
Once the appropriate tubing has been attached from a port to detector, the exiting flow from each of the detectors can be adjusted to the required amount. A simple way to measure the amount of flow exiting from each detector is to weigh the amount of mobile phase that elutes through each port over a given period of time. The percentage flow can thus be determined, and the flow ratios can be adjusted by either shortening or lengthening the tubing attached to the outlet line on the detectors accordingly to suit the requirements of the detectors of choice. Different detectors have different requirements of flow, for example, the flow cell of a fluorescence detector (FLD) is not flow rate limited, but care must be taken to avoid over-pressurization of the flow cell. Hence the control of flow through the FLD is usually attained by adjusting the pressure drop across the other detectors and the remainder of the flow then passes through the FLD. A detector that is sensitive to the amount of flow that is delivered is the MS. Generally, current high end mass spectrometers can readily process around 1 to 1.5 ml/min of moderately aqueous mobile phase. Above this flow rate, flooding of the source may make the MS inoperable. However, detection sensitivity in most mass spectrometers is benefited by using lower flow rates; hence the PSF flow splitting capabilities are extremely useful for applications involving MS detection. High column volumetric flow rates can be utilized, but with low volume loads transported to the MS detector. Tuning of the flow to the MS detector, however, must be made by adjusting the pressure drop prior to the MS detector, rather than post MS. Here, the use of narrow bore tubing (0.1 mm i.d.) is very useful, as the pressure can be readily adjusted without adding inappropriate dead volume.
Depending on the type of detector the adjustment of the segmentation ratio can be done either pre or post detector. If a non-destructive detector, such as UV-Vis is used, the flow percentage would be measured and tuned post detector. If a single destructive detector is used in the multiplex set up the flow percentage is determined by back calculating with respect to other port flow percentages. If a reagent based detector is used such as DPPH•, the flow percentage is measured post detector without the addition of reagent; and if two or more destructive detectors are used, then the flow ratio is measured pre-detector. Detection systems that may require additional instrumentation, such DPPH•, will have extra system pressure that may alter the flow percentage once attached to the detection system. Therefore, careful consideration should be paid to system pressure of a destructive detector, when adjusting flow percentage pre-detector. Irrespective of the flow ratio that is set through any of the ports, quantitative information should be obtained through appropriate standardization. Once the flow ratios are set, however, they are robust, and they do not change even under gradient elution conditions7,
The detailed video protocol accompanying this manuscript is intended to show how to use and tune a PSF column functioning in a multiplexed mode of detection.
Protocol
Note: This protocol contains instructions on how to use a PSF column on a HPLC system coupled with multiple detectors for multiplexed detection. The protocol has been written assuming the reader has basic knowledge and experience in chromatography and various HPLC detection methods.
Caution: Please refer to material safety data sheets (MSDS) for all materials and reagents before use (i.e., MSDS for methanol). Ensure the use of all appropriate safety practices when handling solvents and High Performance Liquid Chromatography (HPLC) eluent. Ensure appropriate use of engineering controls of HPLC, analytical balance and detector instrumentation, and ensure the use of personal protective equipment (safety glasses, gloves, lab coat, full length pants, and closed-toe shoes).
1. Setup of HPLC Instrument
- Prepare the HPLC instrument with ultrapure water (e.g., 100% Milli-Q water) for line A and 100% methanol for line B as the mobile phase and purge the pumps as per manufacturer requirement. If one of the detectors used is MS, as it is here, add 0.1% formic acid to both mobile phases A and B.
- Set up the HPLC instrumental components and detectors as illustrated in Figure 2. This requires convenient placement of detectors relative to the column so as to minimize the dead volume between detector and column. Flexibility in the HPLC system configuration is desirable.
2. Setup of UV-Vis and MS Detectors
- Set the UV-Vis detector to the desired wavelength dependent on the sample of interest (e.g., 280 nm).
- Set the MS detector in positive mode for Total Ion Count (TIC) analysis using Full Scan detection method. Also adjust the following MS parameters accordingly: vaporizer temperature 500 °C, capillary temperature 350 °C, sheath gas set at a rate of 60 units, auxiliary gas flow 40 and sweep gas flow at 5 units, and spray voltage 3.5 kV. These settings can be adjusted later for specific user requirements dependent on the sample being analyzed.
3. Preparation of 2,2-Diphenyl-1-picrylhydrazyl Radical (DPPH•) Reagent Setup of DPPH• System Detector
- Weigh 25 mg of DPPH• and dissolve in 250 ml of methanol in a volumetric flask.
- Add 250 µl of formic acid to the DPPH• reagent. Cover the flask in foil to prevent exposure to light.
- Sonicate the flask containing the DPPH• reagent for 10 min.
- Purge the DPPH• pump with the prepared DPPH• reagent as per manufacturer's requirement.
- Set up the DPPH• system according to Figure 2 by attaching the pump line to the inlet of a T-piece.
- Attach a 100 µl reaction coil to the outlet of the T-piece and attach the other end of the reaction coil to the detector.
- Encase the reaction coil in a column heater and set the column heater temperature to 60 °C.
- Set the DPPH• UV-Vis detector to 520 nm.
4. Setup of PSF Column
- Connect the inlet of the PSF column to the HPLC instrument.
- Connect the central port to the MS detector, using a 15 cm length of 0.13 mm i.d. tubing.
- Connect a peripheral port to the UV-Vis detector using a 15 cm length of 0.13 mm i.d. tubing.
- Connect another peripheral port to the T-piece of the DPPH• detection system using a 15 cm length of 0.13 mm i.d. tubing.
- Block the unused peripheral port using a column stopper.
- Bring the flow rate of the HPLC pump to 1 ml min-1 at 100% line B – 100% Methanol (0.1% formic acid).
- Equilibrate the column with the 100% methanol mobile phase for 20 min for a 4.6 mm i.d. x 250 mm length column. This time is scaled according to the dimensions of other columns the user may employ.
5. Tuning the PSF Column for Multiplexed Detection
- Measure the mass of at least two empty collection vessels (one for the port connected to the UV-Vis detector and one for the DPPH• detector) using an analytical balance.
- Collect mobile phase from the UV-Vis and the DPPH• ports into two separate, pre-weighed collection vessels (5.1). Record the period of time for the collection. Collect at least 500 mg of solvent in each vessel.
- Weigh the collection vessels and determine the mass of mobile phase. Given the density of methanol is 0.791 g ml-1, determine the volume of mobile phase collected from each port.
- By difference, that is, nominal pump set flow rate minus the flow rate through the DPPH• and UV-Vis detector flow ports, determine the flow rate to the MS detector. Express each flow proportion as a percentage of the total flow.
Note: Ideally, the flow percentages are: to the MS is 18% of the total flow rate, to the UV-Vis 22%, to the DPPH• detector 60%. - If not, adjust the flow percentages by changing the back pressure on the UV-Vis detector. For example, if the flow to the UV-Vis is too high, decrease the proportion by adding add a 15 cm section of 0.13 mm i.d. tubing to the outlet of the UV-Vis detector. Then repeat steps 5.1 to 5.5.
6. Final Setup Conditions
- Set the flow rate of the DPPH• reagent pump to the same flow rate exiting the outlet port connected to the DPPH• detector.
Note: The PSF column multiplexed with UV-Vis, DPPH• and MS is now ready for analysis.
Representative Results
A multiplexed HPLC analysis was carried out using an AFT column in PSF mode (Figure 1) and set up as illustrated in Figure 2. This type of setup allowed a coffee sample to be analyzed simultaneously using UV-Vis, DPPH• and MS in Total Ion Count (TIC) mode. The compounds of the coffee sample that responded to DPPH• could then be easily matched up to the UV-Vis and MS – TIC responses based on the alignment of retention time as illustrated in Figure 3, since the chromatograms were recorded simultaneously. Where a positive response was seen from the MS-TIC detector, the molecular mass of the peak was recorded. Table 1 lists the retention times of the DPPH• peaks, and the response of such peaks in the UV-Vis and/or the MS detector, which thus provided the molecular mass. The ease in matching peaks between different detection processes allowed for a fast and more efficient form of screening and characterization for a complex sample, such as coffee.

Figure 1. An image of Active Flow Technology column compared to a conventional column. The AFT column is fitted with a four port outlet fitting that houses the annular frit enabling peak sampling across the radial cross section of a sample band. Please click here to view a larger version of this figure.
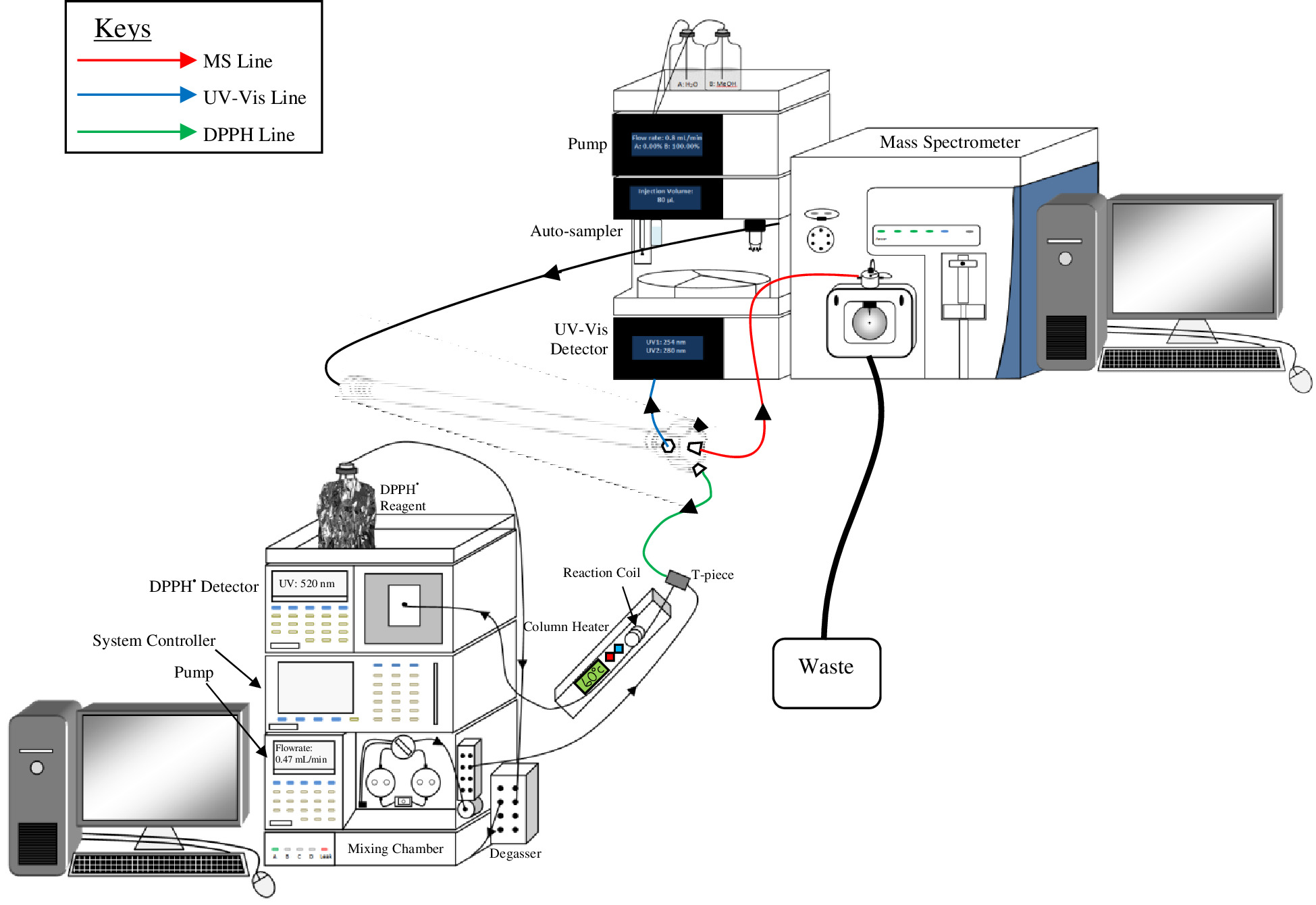
Figure 2. An example illustration of a multiplexed HPLC arrangement, using a PSF column with DPPH•, UV-Vis and MS detectors. Each detector is attached to a separate outlet port. In this case the MS is used for quantification and is set to collect sample from the radial central outlet port. Please click here to view a larger version of this figure.
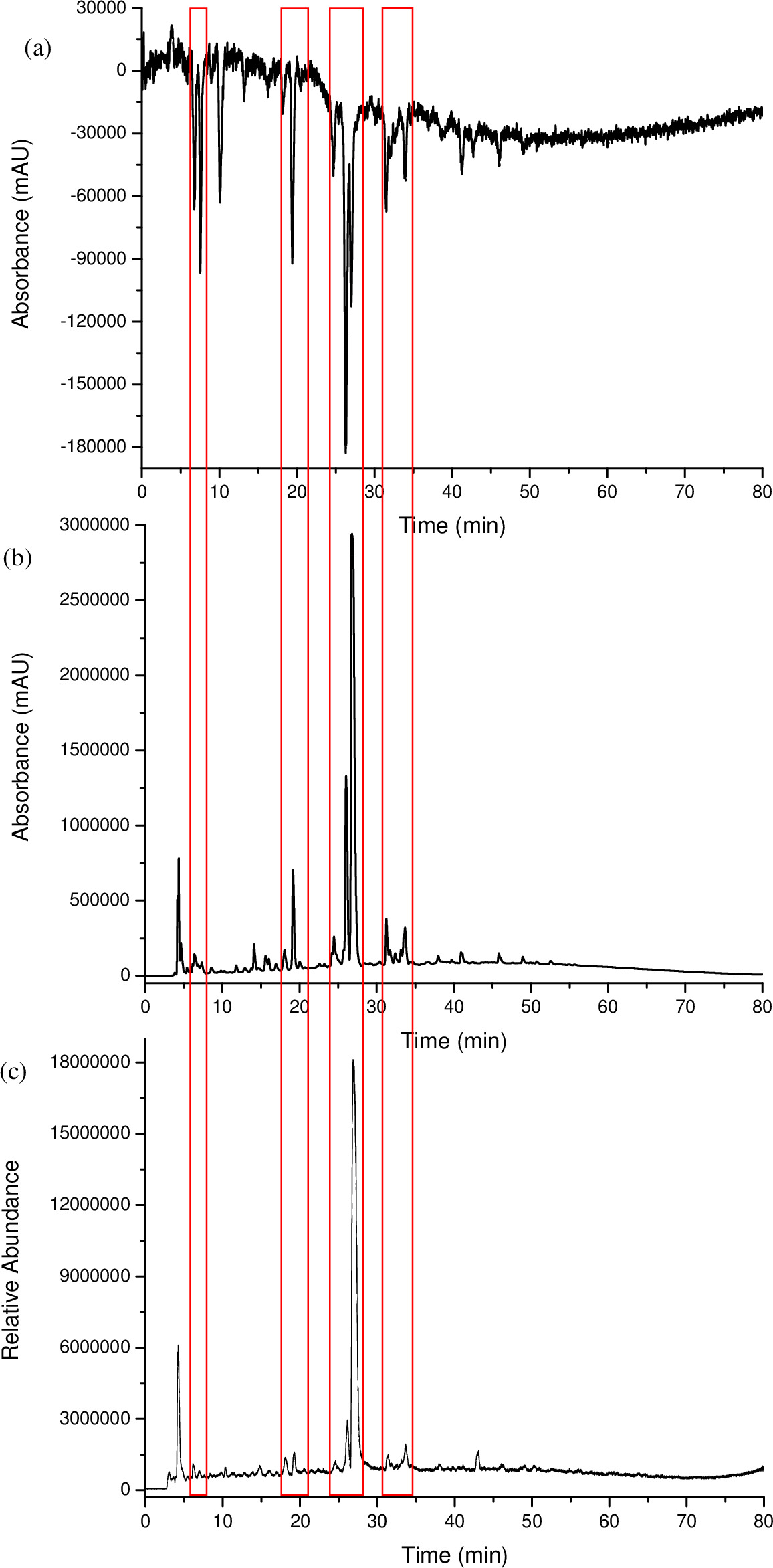
Figure 3. The chromatograms of a coffee sample analyzed via a multiplexed HPLC system, using a PSF column with DPPH•, UV-Vis and MS detectors: (a) DPPH• 520 nm, (b) UV-Vis 280 nm, (c) MS – TIC. Each detector trace is perfectly coincident in time, so no offset adjustment is required in order to compensate for dead time between each detector. Please click here to view a larger version of this figure.
| Coffee DPPH• Peak Response and Mass | |||||
| DPPH• Peak | Retention Time | DPPH• | UV-Vis | MS – TIC Response | Mass |
| (min) | Response | Response | |||
| 1 | 6.74 | Yes | Yes | Yes | 123.84 |
| 2 | 7.54 | Yes | Yes | Yes | 125.83 |
| 3 | 8.94 | Yes | No | No | – |
| 4 | 10.05 | Yes | No | Yes | 135.83 |
| 5 | 13.15 | Yes | No | No | – |
| 6 | 16.22 | Yes | No | No | – |
| 7 | 18.14 | Yes | Yes | Yes | 126.82 |
| 8 | 19.4 | Yes | Yes | Yes | 162.81 |
| 9 | 20.46 | Yes | Yes | Yes | 187.89 |
| 10 | 24.71 | Yes | Yes | Yes | 162.81 |
| 11 | 26.27 | Yes | Yes | Yes | 162.8 |
| 12 | 26.97 | Yes | Yes | Yes | 194.87 |
| 13 | 31.84 | Yes | Yes | Yes | 162.8 |
| 14 | 32.02 | Yes | Yes | Yes | 162.78 |
| 15 | 32.56 | Yes | Yes | Yes | 176.82 |
| 16 | 33.94 | Yes | Yes | Yes | 176.83 |
| 17 | 41.26 | Yes | Yes | No | – |
| 18 | 42.72 | Yes | No | Yes | 284.93 |
| 19 | 46.07 | Yes | Yes | Yes | 190.83 |
| 20 | 49.21 | Yes | Yes | Yes | 162.75 |
Table 1. Detected DPPH• peaks response to UV-Vis and MS – TIC.
Discussion
This study involves the characterization and profiling of coffee using HPLC with multiplexed detection employing a parallel segmented flow (PSF) column. Multiplexed HPLC using PSF columns enables the characterization and identification of key chemical entities by reducing the data complexity of the sample whilst obtaining a greater degree of molecule-specific information within a fraction of the time it takes using conventional multi-detection processes. The PSF column not only allows a platform for multiplexed detection, but also the combination of both destructive and non-destructive detectors, without additional dead volume and tubing. DPPH•, UV-Vis and MS (TIC) were multiplexed for the analysis of espresso coffee.
A 4-port PSF column was utilized for the multiplexed analysis of coffee using the three different detectors. The central port of the PSF column outlet was connected to the MS detector and two of the three peripheral ports were connected to either a DPPH• detection system or a UV-Vis detector. The third available peripheral port was not used and therefore blocked with a column stopper, but this could have been used for the collection of sample, or for another detector. The tuning process of this multiplexed set up was specific to the detector, where measurement of segmentation flow occurred post-detector for UV-Vis and DPPH• detection systems. Since the MS is a destructive detector, the flow percentage was determined by difference (total nominal flow minus the flow from the other outlet ports).
In this study, the coffee analysis with the DPPH• reagent resulted in 20 well resolved peaks, 13 of which also showed a response in the UV-Vis and MS detectors. Figure 3 compares the chromatograms obtained by each detector. There are four regions that have the same chromatographic profile within each chromatogram, indicated by the red boxes. In these boxes, where UV-Vis and MS showed little response to these compounds, there was a strong response from the DPPH• detector. Four components that responded to the DPPH• reagent were not detected either by the UV-Vis or MS detectors and three of the components that responded to the DPPH• reagent gave no UV-Vis or MS response at all. The molecular mass of the components that responded to the MS are recorded in Table 1. The component that eluted at 10 min had a strong DPPH• response, with no response from the UV-Vis detector and only a very small response from the MS detector.
The coupling of an AFT column in PSF mode gave the opportunity to run multiple detectors in multiplexed mode, where all detectors regardless of HPLC-detector requirements were run simultaneously in a single injection and separation of this complex sample. The multi-port end fitting design of the AFT column offers the added benefit of providing opportunities for multiplexing detection processes, yielding detailed sample information and absolute reliability in the assignment of components between each detection mode. Multiplexed detection with PSF columns provided a large amount of sample information, three detectors operated simultaneously within the run time required for a single detector. The exact match of retention time of peaks within each detection mode was possible. Two of the detectors used were destructive detectors.
The multiplexing capabilities of a PSF column is, however limited by the available HPLC instrumental components and detection instrumentation. The technique requires multiple detection methods and the necessary add-ons for each specific mode of detection, i.e., pumps, reaction coils, heaters etc. The primary benefit of multiplexing detection using a PSF column is the reduction in analysis time by up to four fold (if 4 detectors are used), which minimizes sample variability between analyses for each single mode of detection. Furthermore, assigning the component relationships within the sample detected from one detector to another is far simpler, and prone to less error than if each detector were employed separately. This avoids mismatch in component assignments, which is often a problem in the analysis of complex samples.
It is important to reiterate that quantification should be made based on the detector located on the radial central outlet port since separation efficiency here is the highest, hence, peak tailing effects are minimal and quantification is thus the most precise. The flow segmentation has been shown to be robust through analyses, but even so, it is good laboratory practice to maintain regular standardization. Hence in any quantitative work it is important to run standards as appropriate. If a detector is used purely as a means of understanding the sample complexity through visual depiction of sample constituents, quantification may not be required, as is the case here for the antioxidant response detection protocol.
We have demonstrated here an example of triple detection, UV, MS and antioxidant responsive detection. Multiplexed detection systems using AFT columns can be used in almost any situation where an analysts requires multidimensional sample information and this involves multiple detection protocols. Using AFT columns will greatly simplify and speed the process of sample characterizations.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by UWS and ThermoFisher Scientific. One of the authors (DK) acknowledges the receipt of an Australian Postgraduate Award.
Materials
| HPLC instrument | Multiple detectors of choice for multiplexed detection. Detectors of choice may require additional instrumentation i.e. pump. | ||
| Parallel Segmented Flow HPLC column | Thermo Fisher Scientific | Not Defined | Soon to be commercialised |
| Methanol | Any brand | HPLC Grade | |
| PEEK tubing | Any brand | Various lengths and i.d. | |
| Column stoppers | Any brand | For blocking unused peripheral ports. | |
| PEEK tube cutter | Any brand | ||
| Analytical Scale Balance | Any brand | ||
| Stop watch | Any brand | ||
| Eluent collection vessels | Any brand | 1-2 mL Sample vials can be used as eluent collection vessels |
Referenzen
- Camenzuli, M., Ritchie, H. J., Ladine, J. R., Shalliker, R. A. The design of a new concept chromatography column. Analyst. 136 (24), 5127-5130 (2011).
- Camenzuli, M., Ritchie, H. J., Ladine, J. R., Shalliker, R. A. Enhanced separation performance using a new column technology: Parallel segmented outlet flow. J. Chromatogr, A. 1232, 47-51 (2012).
- Camenzuli, M., Ritchie, H. J., Ladine, J. R., Shalliker, R. A. Active flow management in preparative chromatographic separations: A preliminary investigation into enhanced separation using a curtain flow inlet fitting and segmented flow outlet. 35 (3), 410-415 (2012).
- Camenzuli, M., Ritchie, H. J., Shalliker, R. A. Gradient elution chromatography with segmented parallel flow column technology: A study on 4.6mm analytical scale columns. J. Chromatogr., A. 1270, 204-211 (2012).
- Camenzuli, M., Ritchie, H. J., Shalliker, R. A. Improving HPLC separation performance using parallel segmented flow chromatography. Microchem. J. 111, 3-7 (2013).
- Shalliker, R. A., Ritchie, H. Segmented flow and curtain flow chromatography: Overcoming the wall effect and heterogeneous bed structures. J. Chromatogr, A. 1335, 122-135 (2014).
- Camenzuli, M., Ritchie, H. J., Shalliker, R. A. Evaluating active flow technology HPLC columns as a platform for multiplexed detection. Microchem. J. 110, 473-479 (2013).
- Camenzuli, M., et al. Parallel segmented outlet flow high performance liquid chromatography with multiplexed detection. Anal. Chim. Acta. 803, 154-159 (2013).
- Shalliker, R. A., Camenzuli, M., Pereira, L., Ritchie, H. J. Parallel segmented flow chromatography columns: Conventional analytical scale column formats presenting as a ‘virtual’ narrow bore column. J. Chromatogr., A. 1262, 64-69 (2012).
- Soliven, A., et al. Improving the performance of narrow-bore HPLC columns using active flow technology. Microchem. J. 116, 230-234 (2014).
- Camenzuli, M., Ritchie, H. J., Dennis, G. R., Shalliker, R. A. Parallel segmented flow chromatography columns with multiplexed detection: An illustration using antioxidant screening of natural products. Microchem. J. 110, 726-730 (2013).

