A Human Fallopian Tube Model for Investigation of C. trachomatis Infections
Summary
We describe an ex vivo infection model for visualisation of direct interactions from bacterial pathogens with human fallopian tube cells. The whole organ tissue model was established to investigate C. trachomatis induced pathology to the female fallopian tube under “life-like” conditions.
Abstract
Genital tract infections with Chlamydia trachomatis (C. trachomatis) are the most frequent transmitted sexually disease in women worldwide. Inefficient clearance or persistence of the pathogens may lead to ascending infections of the upper genital tract and are supposed to cause chronic inflammatory damage to infected tissues 1,2. As a consequence, severe clinical sequelae like pelvic inflammatory disease (PID), tubal occlusion and infertility may occur 3,4.
Most of the research with C. trachomatis has been conducted in epithelial cell lines (e.g. HEp-2 cells and HeLa-229) or in mice. However, as with cell- culture based models, they do neither reflect the physiology of native tissue nor the pathophysiology of C. trachomatis genital tract infections in vivo 5. Further limitations are given by the fact that central signaling cascades (e.g. IFN-γ mediated JAK/STAT signaling pathway) that control intracellular chlamydial growth fundamentally differ between mice and humans 6,7. We and others therefore established a whole organ fallopian tube model to investigate direct interactions between C. trachomatis and human fallopian tube cells ex vivo 8,9.
For this purpose, human fallopian tubes from women undergoing hysterectomy were collected and infected with C. trachomatis serovar D. Within 24 h post infection, specimen where analyzed using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) to detect Chlamydia trachomatis mediated epithelial damage as well as C. trachomatis inclusion formation in the fallopian tissue.
Protocol
1. Preparation of Chlamydia trachomatis Serovar D Stock
- Infect confluent 175 cm2 cell culture flask containing HeLa-229 cells with 4 ml C. trachomatis D in SPG buffer.
- Incubate for one hour at 37 °C during constant shaking.
- Add 20 ml DMEM with 10% FCS and 240 μl Cycloheximide.
- Incubate in a humidified incubator for 48 h at 37 °C and 5% CO2.
- Add 5 ml sterile glass beads, strong shake to detach and destroy C. trachomatis harboring HeLa-229 cells.
- Collect supernatant and add another 5 ml of sterile glass beads.
- Rock three times for 1 min to destroy all HeLa-229 cells and release C. trachomatis.
- Centrifuge for 5 min at 1000 rpmi and 4 °C to get rid of cellular debris.
- Collect the supernatant for infection of 8 to 10 175 cm2 cell culture flasks confluent grown with HeLa 229 cells.
- Incubate every flask with 4 ml SPG buffer and 4 ml supernatant from previous infection for one hour at 37 °C with constant shaking.
- After one hour add 15 ml DMEM with 10% FCS and 230 μl cycloheximide followed by 48 h incubation at 37 °C and 5% CO2.
- Add 5 ml sterile glass beads, shake to detach and destroy C. trachomatis harboring HeLa-229 cells.
- Collect the supernatant and add another 5 ml of sterile glass beads.
- Rock three times for 1 min to destroy all HeLa-229 cells and release C. trachomatis.
- Centrifuge for 5 min at 1000 rpmi at 4 °C to get rid of cellular debris.
- Collect the supernatant.
- Fill 40 ml of supernatant in 50 ml Falcon tubes, centrifuge for 99 min with 11000 rpmi.
- After centrifugation discard the supernatant and wash the pellet with 1 ml SPG buffer.
- Homogenize the pellet in 1 ml SPG and aliquot 20 μl in a 1, 5 ml Eppendorf tube, store at -70 °C until use. Biological activity of the prepared stock was confirmed in an established HeLa-229 cell infection model.
2. Preparation of Human Fallopian Tube
- Store human fallopian tubes (HFT) from women undergoing hysterectomy immediately after surgery in RPMI containing 5% FCS without antibiotics in 4 °C until preparation. The protocol was approved by the ethical committee of the University of Lübeck (09-153). Women included in the study (age 34 until 53) had no history of prior C. trachomatis infection. Tissue of the Fallopian tubes was collected at peripartum sterilisation on maternal request during caesarean sections or during hysterectomies due to symptomatic uterine fibroids in the luteal phase. All HFT were tested negative for C. trachomatis by PCR.
- Dissect the HFT in a Petri dish containing RPMI with 5% FCS without antibiotics. During the whole processing the tissue should be submerged in media to prevent drying.
- Cut off and discard connective tissue and tissue destroyed during surgery.
- After dissection open the HFT carefully with a small scalpel.
- For further experiments prepare pieces of tissue in the size of 0.5 cm x 0.5 cm up to 1 cm x 1 cm.
- For infection store HFT specimen in 1 ml RPMI containing 5% FCS without antibiotics. HFT specimens were infected with 5.5×105 IFU (inclusion forming units) of C. trachomatis.
- Incubate HFT specimen in 37 °C, 5% CO2 and 21% O2 as well as in 37 °C, 5% CO2 and 2% O2 for 24 h.
- After incubation time collect specimen and store in Monti’s fixative for at least three days at 4 °C until SEM/TEM preparation.
3. Preparation of HFT Specimen for TEM
- For TEM remove specimen from Monti’s fixative and cut 2 x 2 mm to 4 x 5 mm pieces. The remaining tissue can be used for SEM.
- Wash pieces with sodium cacodylate buffer, pH 7.35 for 1 h.
- Incubate in 1% (w/v) osmium tetroxide in aqua dest. overnight.
- Remove excess osmium tetroxide by washing 6×5 min in sodium cacodylate buffer, pH 7.35.
- Remove water by incubating in increasing concentrations of ethanol in water (v/v) (30% for 2 h, 40% for 2 h, 50% for 2 h, 60% for 2 h, 70% over night, 80% for 2 h , 90% for 1 h, 95% for 1 h, 100% for 1 h.
- Wash out ethanol for 2 x 15 min in propylene oxide and subsequently transfer to a mixture of 50% (v/v) freshly prepared araldite (including all components) in propylene and incubate overnight.
- Transfer to freshly prepared araldite (including all components) for at least 1 h.
- Transfer to flat embedding molds and fill with araldite.
- Let araldite harden for at least 48 h at 60 °C
- After trimming of embedded sample cut semi thin sections (700 nm) on an ultramicrotome using glass knifes or a histo diamond knife and stain with Richardson’s stain.
- Cut ultra-thin sections (approx. 70 nm) using a diamond knife and transfer to copper grids (e.g. 150 square mesh).
- Stain ultra-thin sections with 0.5% (w/v) uranyl acetate in aqua dest. followed by 3% (w/v) lead citrate in aqua dest. in an automatic section stainer. Alternatively, sections can be stained manually by leaving grids 15 min on a saturated centrifuged solution of uranyl acetate in aqua dest. followed by 0.3% lead citrate (w/v) in aqua dest.
4. Preparation of HFT specimen for SEM
- For SEM remove specimen from Monti’s fixative, orient with epithelium facing upwards on a 1.5 x 1 cm cork sheet and fix position using insect needles.
- Transfer specimen on cork to suitable metal baskets and wash for 30 min in sodium cacodylate buffer, pH 7.35.
- Remove water by incubating specimen in increasing concentration of acetone/water mixtures (v/v) 30% for 6 h, 40% for 6 h, 60% for 8 h, 70% over night, 80% for 2 h, 90% for 2 h and 100% over night (be extremely cautious to keep specimen continuously submerged).
- Transfer to fresh 100% acetone and dry specimen by critical point drying (temperature 40 °C, pressure 80 bar).
- Remove needles, cork and glue specimen with the epithelium facing upwards on SEM specimen mount equipped with a conductive carbon tab using conductive carbon cement. Use liquid conductive silver to enhance conductivity between the mount and the conductive carbon tab.
- Prepare a conductive platinum, gold or palladium coating of the specimen using a sputter coater.
5. Staining of Semi Thin Sections with Richardson’s Stain
- Let semi thin sections dry on slide.
- Stain sections with staining solution at 60 °C for 1-2 min.
- Wash in aqua dest.
- Dry sections and coverslip.
6. Representative Results
Within the human fallopian tube infection model we were able to visualize differences in the epithelial morphology between the non-infected controls (Figure 1) and the C. trachomatis– infected tubes (Figure 2) under normoxic condition. Characteristic morphological differences implied pathogen- induced swelling and lysis of fallopian tube epithelial cells that could not be detected in non-infected controls. Using semi thin sections and transmission electron microscopy we were able to prove intracellular C. trachomatis inclusion formation in the ex vivo infected human fallopian tube tissue (Figure 3, 5) but not in non-infected controls (Figure 4). As oxygen concentrations in the female genital tract are already low under physiological conditions 11, and further decrease during an inflammatory process we also investigated our model under hypoxic conditions. Preliminary results indicate the model is useful to analyze chlamydial growth and progeny under varying oxygen concentrations. Chlamydia- induced epithelial cell damage has to be separated from artifacts that are mediated through incautious preparation and handling of the fallopian tubes before infection (Figure 6).
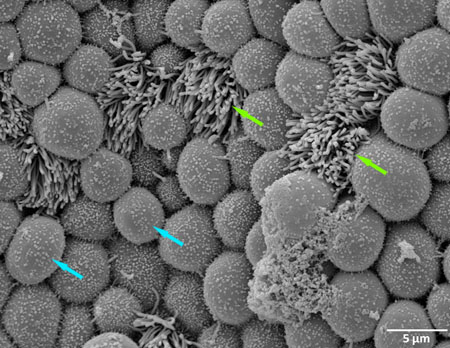
Figure 1. Scanning electron microscopy (SEM) of a non-infected human fallopian after one day incubation in control medium (green arrow show ciliated cell, blue arrow show non- ciliated cell).
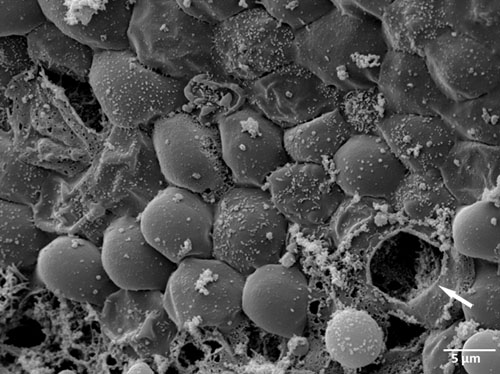
Figure 2. Scanning electron microscopy (SEM) of an ex vivo C. trachomatis infected human fallopian tube one day post infection (white arrow mark ruptured inclusion).
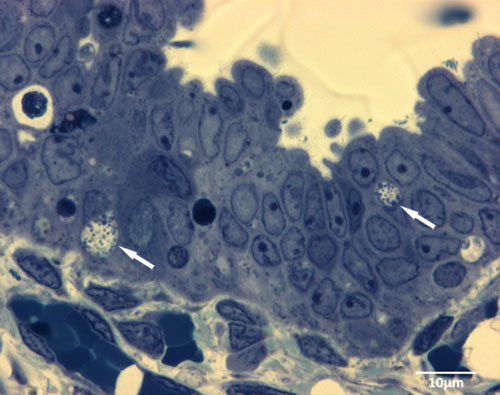
Figure 3. Semi thin sections show typical intracellular inclusions (white arrows) 1d after infection of the human fallopian tube with C. trachomatis ex vivo.
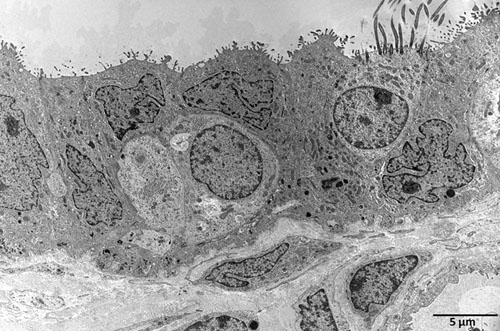
Figure 4. Transmission electron microscopy (TEM) of a non-infected human fallopian tube after one day incubation in control medium.
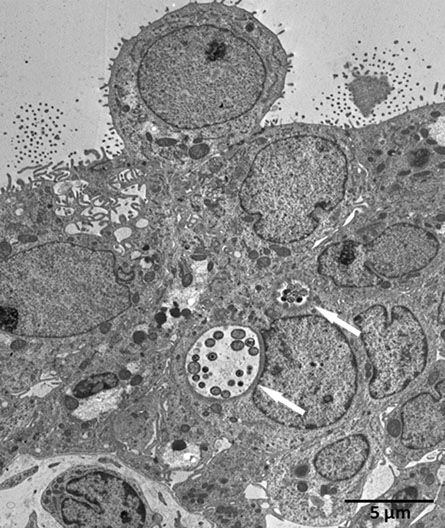
Figure 5. Transmission electron microscopy (TEM) of an ex vivo C. trachomatis infected human fallopian tube one day post infection (white arrows mark chlamydial inclusions).

Figure 6. Scanning electron microscopy (SEM) of fallopian tube epithelium damaged through incautious preparation and handling of specimen (white arrows mark destructed epithelial tissue).
Discussion
Visualization of pathogen- induced harm to the infected tissue is arduous and often limited to experimental procedures in mice. We established an ex vivo infection model in human fallopian tubes to analyze C. trachomatis infections of the upper female genital tract. By the use of this method, we were able to visualize C. trachomatis– induced damage to human fallopian tube epithelium within one day post infection. Cellular swelling and lysis were typical morphological changes that were observed in C. trachomatis– infected fallopian tubes but not in non- infected controls. TEM analyses revealed that chlamydiae implement a typical intracellular developmental cycle within infected tissues, showing large inclusions with re-differentiated elementary bodies but also smaller inclusions with enlarged reticulate bodies that might indicate persistence. However, the morphological picture alone does not suffice to discriminate between replicating and persistent infections, which are characterized by reduced metabolism and increased resistance to antimicrobials 10.
Destruction of the epithelium in infected fallopian tubes is either due to disruption of C. trachomatis– infected epithelial cells after completion of the intracellular developmental cycle and release of infectious elementary bodies or the release of pro- inflammatory cytokines 8. Pathogen- induced tissue damage and host- induced immune reactions against C. trachomatis are supposed to be the major factors leading to loss of epithelial cell function, fibroblast remodeling and finally tubal infertility in vivo 8,11.
One of the major limitations of this model is the absence of inflammatory cells which hampers the analysis of secondary effects to the initial C. trachomatis infection of the fallopian tube epithelium. However, the model is appropriate to investigate different environmental conditions (e.g. oxygen content) and the initial host-immune response in acute C. trachomatis infections 8,9. Further plans are to establish a model for testing of antimicrobials against C. trachomatis in whole human fallopian tubes and to characterize the metabolic states of the different developmental stages observed in the tubal epithelium by 2-photon laser scanning microscopy.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by the DFG-Cluster of Excellence “Inflammation at Interfaces” (RA-If, RA-D). We are grateful for the excellent technical assistance of Kristin Wischnat.
Materials
| Name of the reagent | Company | Catalogue number | Comments |
| DMEM | PAA | E15-843 | |
| RPMI | PAA | R15-802 | |
| FCS | PAA | A15-101 | |
| Cycloheximide | Sigma – Aldrich | ||
| SPG Buffer | various | see recipe below | |
| Monti’s fixative | various | see recipe below | |
| sodium cacodylate buffer | various | see recipe below | |
| Richardson’s stain | various | see recipe below |
Recipes:
1. SPG Buffer
- Add 75 g sucrose.
- Add 2.47 g disodium phosphate.
- Add 0.36 g monosodium phosphate.
- Add 0.72 g glutamic acid.
- Fill to 1 l with aqua dest.
- Adjust pH to 7.3.
2. 0.1 M sodium cacodylate buffer, pH 7.35.
- Add 42.80 g dimethylarsenic acid sodium salt.
- Add 68.46 g sucrose.
- Fill to 1 l with aqua dest. to get 0.2 M sodium cacodylate buffer.
- Before use dilute solution with aqua dest. to desired concentration and adjust pH to 7.35 if necessary.
3. Monti’s fixative
- Add 156 ml 0.12 M sodium cacodylate buffer. 3.2 Add 25 ml 25% glutardialdehyde in aqua dest.
- Add 19 ml 10% paraformaldehyde in aqua dest.
- Add 3 ml 3% CaCl2 in aqua dest.
- Adjust pH to 7.35 and fill with aqua dest. to 312 ml final solution.
4. Richardson’s stain
- Mix equal parts of 1% methylene blue in 1% aqueous borax solution and 1% Azur II in aqua dest.
Referenzen
- Dean, D., Suchland, R. J., Stamm, W. E. Evidence for long-term cervical persistence of Chlamydia trachomatis by omp1 genotyping. J. Infect. Dis. 182, 909-916 (2000).
- Campbell, L. A., Patton, D. L., Moore, D. E., Cappuccio, A. L., Mueller, B. A., Wang, S. P. Detection of Chlamydia trachomatis deoxyribonucleic acid in women with tubal infertility. Fertil. Steril. 59, 45-50 (1993).
- Peipert, J. F. Clinical practice. Genital chlamydial infections. N. Engl. J. Med. 349, 2424-2430 (2003).
- Mardh, P. A. Tubal factor infertility, with special regard to chlamydial salpingitis. Curr. Opin. Infect. Dis. 17, 49-52 (2004).
- Ertel, A., Verghese, A., Byers, S. W., Ochs, M., Tozeren, A. Pathway-specific differences between tumor cell lines and normal and tumor tissue cells. Mol. Cancer. 5, 55 (2006).
- Roshick, C., Wood, H., Caldwell, H. D., McClarty, G. Comparison of gamma interferon-mediated antichlamydial defense mechanisms in human and mouse cells. Infect. Immun. 74, 225-238 (2006).
- Mestas, J., Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731-2738 (2004).
- Hvid, M., Baczynska, A., Deleuran, B., Fedder, J., Knudsen, H. J., Christiansen, G., Birkelund, S. Interleukin-1 is the initiator of Fallopian tube destruction during Chlamydia trachomatis infection. Cell Microbiol. 9, 2795-2803 (2007).
- Roth, A., Konig, P., van, Z. G., Klinger, M., Hellwig-Burgel, T., Daubener, W., Bohlmann, M. K., Rupp, J. Hypoxia abrogates antichlamydial properties of IFN-gamma in human fallopian tube cells in vitro and ex vivo. Proc. Natl. Acad. Sci. U.S.A. 107, 19502-19507 (2010).
- Wyrick, P. B., Knight, S. T. Pre-exposure of infected human endometrial epithelial cells to penicillin in vitro renders Chlamydia trachomatis refractory to azithromycin. J. Antimicrob. Chemother. 54, 79-85 (2004).
- Van Voorhis, W. C., Barrett, L. K., Sweeney, Y. T., Kuo, C. C., Patton, D. L. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect. Immun. 65, 2175-2182 (1997).

