Mass Production of Genetically Modified Aedes aegypti for Field Releases in Brazil
Summary
To achieve population suppression of Aedes aegypti using the RIDL® (Release of Insects carrying a Dominant Lethal) system, large numbers of male mosquitoes need to be released. This requires the use of mass rearing techniques and technology to provide reliable systems to obtain the maximum number of high quality male mosquitoes.
Abstract
New techniques and methods are being sought to try to win the battle against mosquitoes. Recent advances in molecular techniques have led to the development of new and innovative methods of mosquito control based around the Sterile Insect Technique (SIT)1-3. A control method known as RIDL (Release of Insects carrying a Dominant Lethal)4, is based around SIT, but uses genetic methods to remove the need for radiation-sterilization5-8. A RIDL strain of Ae. aegypti was successfully tested in the field in Grand Cayman9,10; further field use is planned or in progress in other countries around the world.
Mass rearing of insects has been established in several insect species and to levels of billions a week. However, in mosquitoes, rearing has generally been performed on a much smaller scale, with most large scale rearing being performed in the 1970s and 80s. For a RIDL program it is desirable to release as few females as possible as they bite and transmit disease. In a mass rearing program there are several stages to produce the males to be released: egg production, rearing eggs until pupation, and then sorting males from females before release. These males are then used for a RIDL control program, released as either pupae or adults11,12.
To suppress a mosquito population using RIDL a large number of high quality male adults need to be reared13,14. The following describes the methods for the mass rearing of OX513A, a RIDL strain of Ae. aegypti 8, for release and covers the techniques required for the production of eggs and mass rearing RIDL males for a control program.
Introduction
Mosquitoes transmit many pathogens that can cause a range of diseases in humans and controlling these mosquitoes has been an ongoing battle for centuries. Strategies to control insects based on chemical and biological methods have had some notable successes, but in many cases control has not been sustainable in the long term. For example, Brazil achieved eradication of Ae. aegypti in the 50s but the mosquito has reinvaded over the last 40-50 years. This can be attributed to many causes, including insecticide resistance, environmental damage, poor control program implementation15-20, and the rapid reinvasion without adequate monitoring or response.
New techniques and methods are being sought to try to win this battle against mosquitoes. Recent advances in molecular techniques have led to the development of new and innovative methods of mosquito control based around the Sterile Insect Technique (SIT)1-3. A control method known as RIDL (Release of Insects carrying a Dominant Lethal)4 is based around SIT, but uses genetic methods to remove the need for radiation-sterilization. RIDL strains have been constructed for several pest species including Ae. aegypti 5-8, A RIDL strain of Ae. aegypti was successfully tested in the field in Grand Cayman9,10; further field use is planned or in progress in other countries around the world. Suppressing mosquito populations using RIDL will require a large number of high quality male adults to be reared3,14.
For an SIT program it is considered desirable to release only males; female mosquitoes bite and transmit disease. In addition, the released sterile males can be ‘distracted’ by the released females reducing program effectiveness. Male-only release was shown to be 3-5 fold more effective than mixed-sex release in large field experiments with irradiated Mediterranean fruit flies21.
In a RIDL mass rearing program, there are several stages to producing the males for release. The first is to produce the eggs required for the release generation (Figure 1). The next stage is to rear the eggs through to pupae or adults, separating larvae from pupae and the male pupae from the female pupae. Large-scale separation of males from females requires a difference between the sexes at a particular life stage suitable for mass sorting22. In Ae. aegypti (and other mosquito species) there is a significant size difference between the male and female pupae which can be exploited for sex separation techniques. The sorted RIDL males are then released in a control program as either pupae or as adults11,12.
The following describes the methods for the mass rearing of OX513A, a RIDL strain of Ae. Aegypti, for release. The methods described cover the techniques required for the production of eggs and RIDL males for a control program.
Protocol
Insectary Requirements
1. Overview
- Mass rearing has several phases, each of which is performed in a distinct area of the mass-rearing unit. Figure 1 shows the main phase of rearing and where they occur in the mass rearing facility.
2. Biosecurity Considerations for the Insectary
- The project was approved by the committee of animal ethics from the University of São Paulo - Brazil and all necessary permits and approvals were obtained.
- The RIDL system uses conditional lethality; a dietary supplement (tetracycline) prevents the expression of the RIDL system allowing the insects to be mass reared; without tetracycline the mosquitoes die. Tetracycline is not found in the breeding sites of Ae. aegypti in the wild so any offspring produced from escapees will not survive. This adds an extra level of security over using wild-type (WT) insects sterilized by other methods (i.e. radiation).
- Use double-door entry, “Authorized Personnel Only” signs and insect screens in the insectary. Any windows should be sealed to avoid contamination of the colony with WT insects and help prevent escapes from inside the insectary.
- Local regulations and authorities may have specific additional or alternative requirements. Contact the relevant authorities for information/guidance at an early stage, and secure all necessary permits and approvals before establishing the RIDL strain in an insectary.
- The mass rearing insectary should only be used for RIDL rearing. No other strains or insects should be present. This is to avoid contamination of the RIDL strain and contamination with pathogens that may affect fitness and survival, reducing the productivity and effectiveness of released males.
3. Insectary Design
- Figure 2 shows a plan of the insectary used at Biofábrica Moscamed in Brasil.
- Temperature of all rooms was 26 °C ± 2) with a relative humidity approximately 70-80% and a 12:12 hr light:dark cycle using fluorescent light strips.
- This blueprint can be modified according to specific space requirements, e.g. the scale of the release will determine overall size. However the egg production, rearing and sorting, Quality Control (QC) and releases should be separated into four distinct areas: Egg Production Colony, Release Generation, QC, and Adult Storage for Release.
- The Egg Production Colony room (approximately 20 m2) is used to produce eggs for the release generation.
- The Quality Control area is where experiments are conducted to check procedures and the fitness of the RIDL line including measurements of hatch rate, pupae numbers, pupa size, and measuring effectiveness of sorting and removing females. Tests are also conducted for WT contamination by checking for the presence of fluorescence marker and phenotype using offset assays measuring lethality.
- The Release Generation room (approximately 36 m2) requires adequate space for trays and the sorting of larvae and pupae. The trays are held in purpose-built aluminum racks. A large work area and sink are required for rearing and sorting and another large sink is required for washing of trays and cages. A filter catch trap has been included in the waste water outlet from the sinks to mitigate the escape of living mosquitoes through the sewer.
- All water used for the trays is disposed through a drain, which has a compartment containing a mesh that prevents the escape of insects in any stage of development.
- An area is required for the storage of adults for release (Adult Storage for Release, Figure 2) containing racking to hold the release devices while the adults mature before release.
Production Methods for Mass Rearing RIDL:
The Egg Production Colony produces the eggs that are used in the Release Generation (Figure 1) to produce adults. There are many similarities in the two rearing methods but also some distinct differences. The rearing processes that are the same for both procedures are described in the Egg Production Colony section only.
Egg Production Colony
4. Larval Production
The Egg Production Colony generates homozygous OX513A RIDL eggs8 for the Release Generation. High quality control ensures viability, fitness and strain integrity of the eggs supplied.
- The stimulus for hatching is submersion in water with a low level of dissolved oxygen. To reduce the oxygen level in the water, heat water until it boils and immediately place 400 ml into 500 ml glass jars (74 mm opening circumference), fasten the lid securely and leave at room temperature for several hours to cool.
- Put 1 g of eggs into a jar of boiled water, reseal and wait for an hour. Transfer the contents to a tray with 2 L water and leave overnight under insectary conditions.
- Place the hatched larvae into a known volume of water and stir using a magnetic stirrer and flea for sufficient time to take aliquots; stirring vigorously or for extended time damages larvae and should be kept to a minimum. The standard volume used to hatch larvae is 1 L, however densities of more than 300 larvae/ml are hard to count and may be damaging. Therefore do not hatch more than about 300,000 eggs/L of water.
- Determine the hatch rate by taking three 1 ml aliquots and placing onto a sheet of absorbent paper, with a grid of 1 cm squares, on top of an absorbent sponge to soak up any excess water. Count the number of hatched and nonhatched eggs from three squares by looking for the missing cap of a hatched egg. A hatch rate around 80-90% is expected.
- Take four aliquots of 1 ml each and place into four black weigh boats (larvae are white and therefore more readily visible against a black background) and count the number of live larvae present by eye; the use of a counter is recommended. The average number of larvae per ml is then be used to calculate the volume of water to add on each tray to get the desired number of larvae per tray.
- There can be large differences in the density at which larvae can be reared between strains and species. It is also desirable to compare RIDL adult male size to wild type males; ideally you want similar to larger sized RIDL males that can compete successfully with wild males. Therefore for each RIDL strain the ideal density of larvae needs to be determined and is a trade-off against space constraints for rearing and the scale of production. Densities ranging from 0.1-2.5 larva/ml we have found to be a good combination of production output and quality; also discussed by Medici23.
- Add the larvae to trays. At our facility we use trays measuring about 53 cm x 37 cm x 8 cm (L x W x H) and the required amount of water to achieve the density of larvae for production.
- Add a stock solution of tetracycline (3 mg/ml in water) to the trays at a 1:100 dilution to obtain the required final concentration of 30 µg/ml.
- Feed larvae with flake fish food (www.sera.de) daily, which has been crushed to a fine powder. Table 1 shows the typical feeding regime in mg of food per larvae per day. Feed larvae until the appropriate day of sorting under the insectary conditions described above.
5. Sorting Larvae from Pupae and Male/Female Pupae
- Sort larvae from pupae 8 days after hatching by separating the larvae from the pupae. Then sex-sort the pupae for males. A device known as a plate separator24,25 can sort larvae from male pupae and from female pupae. Figure 3 illustrates the use of this plate separator to separate larvae from male and from female pupae.
- Using a measuring spoon with a mesh on the bottom, calibrate it with 500 pupae or more and use it to set up cages and estimate the total amount of males and females pupae produced.
- On the first day of sorting (day 8) most of the pupae are males, so place the sorted larvae back into a tray and rear through to the following day when most of the pupae are females (Figure 4).
- Repeat the sorting process each day as described in steps 2.1-2.3 until all the larvae have pupated or until the end of a working week.
- Place 1,000 male pupae (using the measure spoon) in a cage and on day 9 add 3,000 female pupae to the same cage (we use 30 cm high and 30 cm diameter PVC pipes adapted with mesh top and a netted access hole, but 30 cm x 30 cm x 30 cm plastic cages are also available from BugDorm).
- Allow adults to mate for at least 2 days and provide them with sucrose solution (10%) on wet cotton ad libitum before blood feeding. Collect eggs for two weeks (~two gonotrophic cycles) with two blood feeds a week. This yields an average 143,000 eggs from a cage of 3,000 females (an average of 48 eggs/female). For production of 4 million eggs a week about 28 cages need to be set up each week.
6. Blood Feeding
- An aluminum plate feeding system supplies cages with blood twice a week. A pocket of blood is created on side of an aluminum plate (10 cm x 10 cm x 3 mm) with Parafilm (Figure 5). To encourage feeding, blood is warmed by placing a heated bean bag on top of plate feeder. The bean bag consisted of approximately 250 g of wheat grains in a cloth bag, which is heated for about 10 sec in a microwave. The blood we use for feeding is from a local abattoir; as these animals (mainly goats and sheep) are for human consumption they are tested to avoid the presence of pathogens.
- Three days after blood feeding, an oviposition site for the females to lay their eggs is provided. The oviposition site is a round plastic container filled to about ¼ with water and filter paper covering the inside. Two days later the oviposition site is removed. For maximum egg production, make sure the filter paper covers the entire available surface inside the container and remains wet.
- Remove the egg paper and place onto absorbent paper to dry under insectary conditions; eggs require a period of conditioning at high humidity (over 70%) of at least 48 hr after being laid. Eggs can be left in the insectary for up to 3 months with minimal decrease in hatch rate, as long as the humidity is kept high25.
- The egg production colony needs to be of sufficient size to provide the number of eggs required on a weekly basis for the release program. The facility described above can produce approximately 4 million eggs with 28 cages set up weekly. It is recommended to keep enough eggs stored to guarantee at least 4 weeks of eggs for Release Generation and Egg Production Colony.
Release Generation
7. Larval Production
The hatching, rearing and sorting processes for the release generation are identical to the previously described method for the Egg Production colony. However, the scale of production is much larger and this requires more trays and more effort.
8. Sorting Larvae, Male Pupae, and Female Pupae
- The sorting of larvae, male pupae and female pupae is identical to method described for Egg Production.
- After sorting the male pupae it is important to check for female contamination before release; there should not be more than 1% females present. Take three aliquots of 500 pupae (three random pupae measuring spoons) and count the number of female pupae present. Females can be identified as they are generally larger than males and from differences in the genital lobe shape (Figure 6)27. If there is greater than 1% females they should be resorted and checked again.
- Use only the males from day 8 and 9 for release; autoclave any remaining larvae and pupae. In Brazil, this waste must be treated the same way as medical waste but regulations may differ in other countries. Do not use female pupae and larvae from the Release Generation for the Egg Production Colony because of differences in quality control, rearing and contamination risks from larger numbers, and lower quality control.
Adult Storage for Release: Place males into release devices to emerge and mature before release. The details of the release devices are not covered in this method. However the capacity of the release devices we use is around 1,000 males. The release method details (release device and release system) are not covered in this method.
Representative Results
The expected pupation results for production are shown in Figure 4. The males pupate first, peaking on day 8 and the females peak on day 9 after hatching. It is important to measure this pupation curve to know the best time to sort males from females.
Results from over 6 months of sorting male and female pupae show that female contamination on average is 0.02% (Figure 5; SEM = 0.004%). This contamination rate represents only 400 females released over one month of releases (approx. two million males). Current egg production per week is 4 million. Of these, 3.5 million are hatched for egg colony and production with the remainder stored for backup. Approximately 11% of eggs are used for egg production colony and remainder for release generation. Egg hatch rate averages 87.3% (SEM = 0.5%) and yield of male pupae from L1 larvae averages 29.5% (SEM = 1.2). Two million larvae are reared every week for the Release Generation colony producing around 571,000 male pupae (SEM = 14,000). Pupae and adult mortality during emergence and release averages 5%, resulting in approximately 543,000 (SEM = 13,000) released adult male mosquitoes per week. It is anticipated current production of 4 million eggs/week could be reduced to ~3 million/week with further optimization to reduce loss from wastage and storage. In total 6 staff are needed for the production described; four working on release generation and the remaining two on the egg colony.
Quality Control
Quality control is essential to maintain the quality of the adults, efficiency of rearing, labor and costs.
Transgene phenotype control
In order to verify RIDL gene expression two controls are performed, firstly to check expression of fluorescence marker and secondly to check expression of RIDL lethality trait in the absence of tetracycline. All larvae should express the fluorescent marker. To check if the fluorescent marker is being expressed as expected, 2,000 first instar larvae are checked under a Leica MZFLIII stereomicroscope with DsRed2 filter (Texas Red) to determine if any nonfluorescent individuals are present. Without tetracycline the RIDL gene is expressed and we expect 3-4% survival to adults8. To check this, an extra tray is set up for each release batch without tetracycline. The only difference to normal rearing is the food is reduced by 2/3 from day 6 because accumulation of excess food due to dead larvae can result in excess bacterial growth.
Rearing control
Four trays from the release generation are randomly chosen to be sorted and counted separately. The number of male and female pupae is recorded from each tray every day (Figure 4). Any deviation from the expected number of males and females indicates a potential issue that could affect production and/or fitness and can be compared to the egg colony to help determine the source of problems.
Pupae Measurements
Pupae size has been shown to be correlated with adult size28. As a quality control for adult size the cephalothorax width is measured of at least 30 male pupae for each sorting day from the Release Generation; for the Egg Production Colony female pupae are also measured. Average cephalothorax width was 1.05 mm (SEM 0.005) for released males and 1.04 mm (SEM = 0.006) and 1.29 mm (SEM = 0.006) for males and females respectively Egg Production, Colony; similar results were obtained for Ae. albopictus mass rearing24.
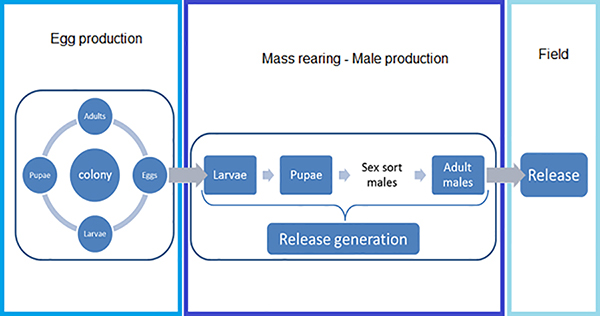
Figure 1. Stages of mass rearing mosquitoes for use in a RIDL/SIT program. The Egg Production Colony has a high level of quality control to ensure the quality of the eggs supplied to the release generation. Eggs are reared through to pupae in the release colony, where the males are sorted from the females. The male adults are then used for the RIDL control program.
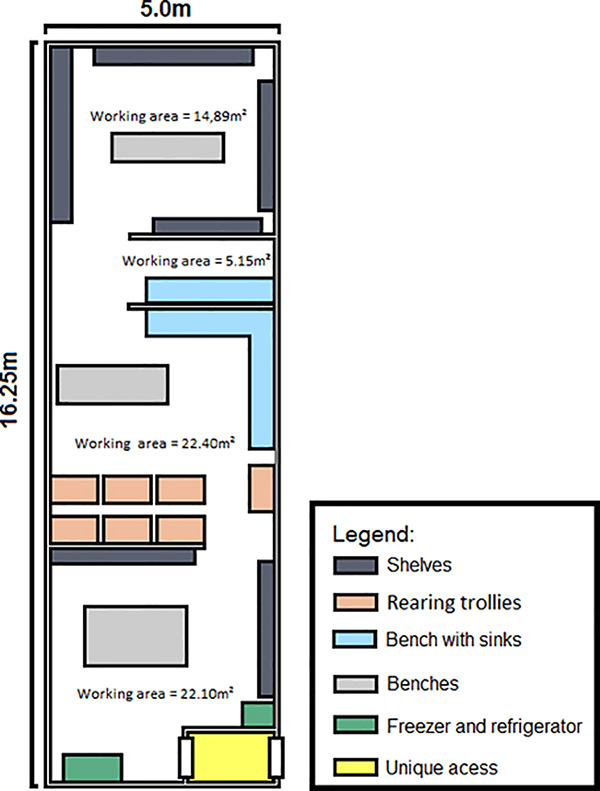
Figure 2. Schematic of the rearing facility for the production of RIDL males for a release program. High quality eggs are continually produced in the Egg Production Colony room and are then reared through to pupae in the Release Generation room. The larvae and pupae are then separated and the pupae sex-sorted for males. The males are then placed into release devices and allowed to mature to adults for release in the adult storage and release room.

Figure 3. Separating larvae, male pupae, and female pupae using a plate separator26. The plate separator uses the size difference between larvae, male pupae, and female pupae to sort these three different life stages; larvae tend to be smaller than male pupae which are in turn smaller than female pupae. The instrument comprises two glass plates; one is fixed to a slanted metal frame and the other sits on top of the first plate and can be moved relative to the first using four adjustment screws (A). The four adjustment screws allow the outer plate to be set at an angle to the back plate so a wedge shaped space is formed between the plates, tapering downwards. Larvae and pupae are poured between the glass plates (B) and gently washed using a water hose (C). By adjusting the angle of the plate, larvae, male pupae and female pupae can be separated (D). With continuous flushing and increasing the plate angle the larvae can be flushed through first (into a sieve), followed by the male pupae and finally the female pupae.
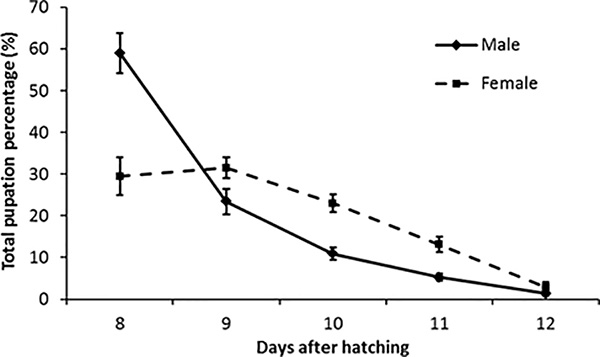
Figure 4. Pupation curves for mass reared RIDL Ae. aegypti. This graph shows the average percentage pupation for mass reared RIDL male and female pupae during 23 weeks observation with ~135,000 pupae recovered per week. Error bars = standard error of mean, n = 23. On the first collection (day 8) we recovered on average 59% male and 30% female pupae from total pupae recovered from trays over 5 days.
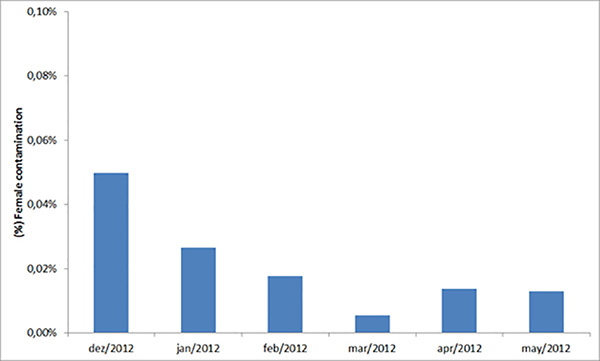
Figure 5. Average female contamination of sorted male pupae. This graph shows the monthly average percentage female contamination during male sorting on day 8 after hatching over a six month period.
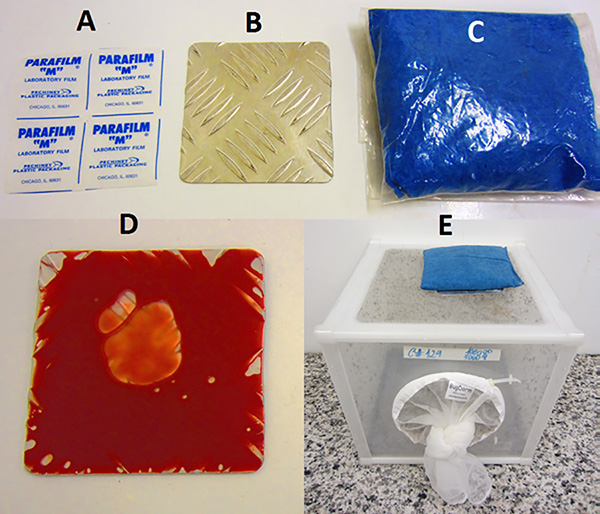
Figure 6. Aluminum plate blood feeder system. The plate (B) is covered with Parafilm (A) and blood pipetted into a pocket and then sealed (D). The plate is placed on a cage and heated by placing a warmed bean bag (C) on top (E).
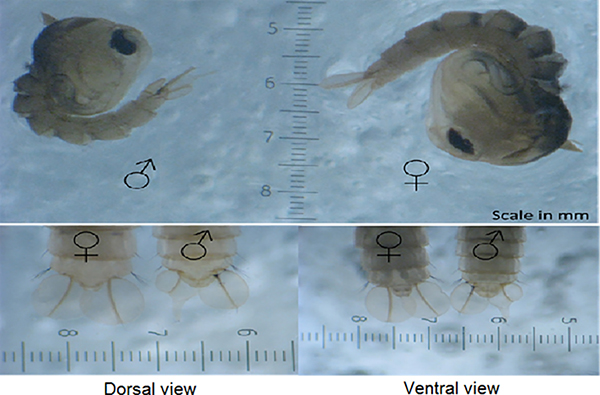
Figure 7. Distinguishing male and female Ae. aegypti pupae. Ae. aegypti pupae can be reliably sexed by the differences in the genital lobe shape (at the end of the pupal abdominal segments just below the paddles). In addition, males also tend to be smaller than females.
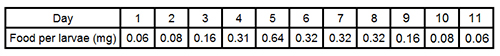
Table 1. General feeding regime for Ae. aegypti RIDL larvae (mg of food per larva per day). To calculate actual amount of food required multiply by the total number of larvae per tray.
Discussion
RIDL is an effective and environmentally safe method of controlling mosquitoes3,29-31. The technique is applicable to an integrated pest management program and most current control methods, including larvicides, breeding site reduction and adulticides are compatible with this technology. This method describes how to produce up to 570,000 RIDL male pupae per week for use in the control of Ae. Aegypti and to our knowledge this is the first description of the production of transgenic mosquitoes on this scale. Some comparable production systems were developed for wild type Ae. aegypti in the 1960s and 70s25, however there has been no comparable production on this scale since then. In Brazil around 11 million males have been released from February 2011 up to February 2012. The numbers of males required for a given area to be controlled is dependent on a number of factors including the size of wild population, dispersal of released males, survival and mating competitiveness of males after release, and environmental conditions. Previous studies have shown that RIDL can reduce a population of mosquitoes by at least 80%9.
A balance is needed between optimizing mass rearing for scale of production and cost vs. quality of males. For example, increasing larval density can increase production capacity by reducing the space required, labor and time to pupation32. However, too high densities of larvae can result in smaller and shorter lived males with reduced mating capacity32,33. Quality of males in relation to a SIT program will ultimately be assessed by the ability of released males to mate with females in field. Extensive field evaluation is required to assess mating competitiveness relative to wild counterparts9,10. This often makes it impractical to evaluate exactly which factors make a ‘high quality’ male mosquito. However, maintaining consistent production and quality (to the extent that can be routinely evaluated) in large-scale mass rearing is paramount. This requires a high level of vigilance and standardization of all process with small fluctuations potentially having a significant impact. The aliquoting of L1 larvae is a critical step and illustrates this point. Aliquoting the correct number of larvae into trays is essential for good quality production. The feeding regime is precisely tailored for specific number of larvae. Too few/many larvae will result in over/under feeding, which influences larval survival, size of pupae and time to pupation. If there is a secret to the art of mass rearing it is to ensure the many small steps in the production cycle are conducted consistently, precisely and with a high level of quality control, as described in this paper.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We would like to thank Biofábrica Moscamed Brasil, Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnologia (CNPq) for financial support. We would also like to thank the following people for their assistance; Miriam dos Santos, Gildeane Silva, Gessilane dos Santos, Fabio Gonçalves, John Paul Oliveira, Luiza Garziera, José Carlos Valença.
Materials
|
Vipan Premium |
Sera GmbH |
190 |
http://www.sera.de/uk/pages/products/product/sera-vipan-3.html |
Required for rearing RIDL larvae |
|
Tetracycline |
Sigma Aldrich |
T7660 |
Required for rearing RIDL larvae |
|
|
Plate separator |
J.W. Hock |
5412 |
http://www.johnwhock.com/download/manuals/instr_5412_separator.pdf |
Separating larvae and pupae |
|
Parafilm M |
Pechiney Plastic packing |
PM-996 |
Cover plate for blood feeding system |
|
|
Rearing pans for Release generation (53 cm x 38 cm x 8 cm) |
Pleion |
0757 |
Larval rearing Release generation |
|
|
Fluorescent scope |
Leica Microsystems |
MZ FLIII |
http://www.leica-microsystems.com/fileadmin/downloads/Leica%20MZ%20FLIII/Brochures/M1-160-0de.pdf |
Viewing fluorescent RIDL larvae |
|
Adult cages |
BugDorm |
DP1000 |
http://bugdorm.megaview.com.tw/bugdorm-1-insect-rearing-cage-30x30x30-cm-pack-of-one-p-29.html |
Cages for Egg production colony |
|
Filter paper |
CELAB |
Filter paper for egg laying |
Referenzen
- Dyck, V., et al. Sterilizing Insects with Ionizing Radiation. Sterile Insect Technique. , 233-268 (2005).
- Dyck, V., Hendrichs, J., Robinson, A. S., Klassen, W., Curtis, C. History of the Sterile Insect Technique.. Sterile Insect Technique. , 3-36 (2005).
- Alphey, L., et al. Sterile-insect methods for control of mosquito-borne diseases – an analysis. Vector Borne Zoonotic Dis. 10, 295-311 (2010).
- Thomas, D. D., Donnelly, C. A., Wood, R. J., Alphey, L. S. Insect population control using a dominant, repressible, lethal genetic system. Science. 287, 2474-2476 (2000).
- Fu, G., et al. Female-specific insect lethality engineered using alternative splicing. Nat. Biotechnol. 25, 353-357 (2007).
- Fu, G., et al. Female-specific flightless phenotype for mosquito control. Proc. Natl. Acad. Sci. U.S.A. 107, 4550-4554 (2010).
- Gong, P., et al. A dominant lethal genetic system for autocidal control of the Mediterranean fruitfly. Nat. Biotechnol. 23, 453-456 (2005).
- Phuc, H. K., et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 5 (11), (2007).
- Harris, A. F., et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 30, 828-830 (2012).
- Harris, A. F., et al. Field performance of engineered male mosquitoes. Nat. Biotechnol. 29, 1034-1037 (2011).
- Bailey, D. L., Lowe, R. E., Dame, D. A., Seawright, J. A. Mass rearing the genetically altered MACHO strain of Anopheles albimanus Wiedemann. Am. J. Trop. Med. Hyg. 29, 141-149 (1980).
- Benedict, M. Q., et al. Colonisation and mass rearing: learning from others. Malar. J.. 8 Suppl 2 (S4), (2009).
- Alphey, L. Re-engineering the sterile insect technique. Insect Biochem. Mol. Biol. 32, 1243-1247 (2002).
- Wise de Valdez, ., R, M., et al. Genetic elimination of dengue vector mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 108, 4772-4775 (2011).
- Macoris Mde, ., L, , et al. Resistance of Aedes aegypti from the state of Sao Paulo, Brazil, to organophosphates insecticides.. Mem. Inst. Oswaldo Cruz. 98, 703-708 (2003).
- Campos, J., Andrade, C. F. Larval susceptibility of Aedes aegypti and Culex quinquefasciatus populations to chemical insecticides. Rev. Saude Publica. 37, 523-527 (2003).
- Gubler, D. J. Resurgent vector-borne diseases as a global health problem. Emerg. Infect. Dis. 4, 442-450 (1998).
- Harris, A. F., Rajatileka, S., Ranson, H. Pyrethroid resistance in Aedes aegypti from Grand Cayman. Am. J. Trop. Med. Hyg. 83, 277-284 (2010).
- Lima, J. B., et al. Resistance of Aedes aegypti to organophosphates in several municipalities in the State of Rio de Janeiro and Espirito Santo. Am. J. Trop. Med. Hyg. 68, 329-333 (2003).
- Paris, M., et al. Persistence of Bacillus thuringiensis israelensis (Bti) in the environment induces resistance to multiple Bti toxins in mosquitoes. Pest Manag. Sci. 67, 122-128 (2010).
- Rendon, P., McInnis, D., Lance, D., Stewart, J. Medfly (Diptera: Tephritidae) genetic sexing: large-scale field comparison of males-only and bisexual sterile fly releases in Guatemala. J. Econ. Entomol. 97, 1547-1553 (2004).
- Papathanos, P. A., et al. Sex separation strategies: past experience and new approaches. Malar. J.. 8 Suppl 2 (S5), (2009).
- Medici, A., et al. Studies on Aedes albopictus larval mass-rearing optimization. J. Econ. Entomol. 104, 266-273 (2011).
- Focks, D. A. An improved separator for the developmental stages, sexes and species of mosquito (Diptera Culicidae).. J. Med. Entomol. 17, 567-568 (1980).
- Fay, R. W., McCray, E. M., Kilpatrick, J. W. Mass production of sterilized male Aedes aegypti. Mosquito News. 23, 210-214 (1963).
- Christophers, S. R. . Aedes aegypti the yellow fever mosquito: Its life history, Bionomics and Structure. , (2009).
- Jones, J. C. A simple method for sexing living Anopheles Larvae (diptera, culicidae). Ann. Entomol. Soc. Am. 50, 104-106 (1957).
- Koenraadt, C. J. M. Pupal Dimensions as Predictors of Adult Size in Fitness Studies of Aedes aegypti (Diptera Culicidae).. J. Med. Entomol. 45, 331-336 (2008).
- Alphey, L., Nimmo, D., O’Connell, S., Alphey, N. Insect population suppression using engineered insects. Adv. Exp. Med. Biol. 627, 93-103 (2008).
- Alphey, N., Bonsall, M. B., Alphey, L. Modeling resistance to genetic control of insects. J. Theor. Biol. 270, 42-55 (2011).
- Atkinson, M. P., et al. Analyzing the control of mosquito-borne diseases by a dominant lethal genetic system. Proc. Natl. Acad. Sci. U.S.A. 104, 9540-9545 (2007).
- Bargielowski, I., Nimmo, D., Alphey, L., Koella, J. C. Comparison of life history characteristics of the genetically modified OX513A line and a wild type strain of Aedes aegypti. PLoS One. 6 (e20699), (2011).
- Bargielowski, I., Alphey, L., Koella, J. C. Cost of mating and insemination capacity of a genetically modified mosquito Aedes aegypti OX513A compared to its wild type counterpart. PLoS One. 6 (26086), (2011).

