Organotypic Collagen I Assay: A Malleable Platform to Assess Cell Behaviour in a 3-Dimensional Context
Summary
A method is described for the preparation of a 3-dimensional matrix consisting of collagen type I and primary human fibroblasts. This organotypic gel serves as a useful substrate to assess invasive cell migration because it mimics basic features of tissue stroma and is amenable to many forms of microscopy.
Abstract
Cell migration is fundamental to many aspects of biology, including development, wound healing, the cellular responses of the immune system, and metastasis of tumor cells. Migration has been studied on glass coverslips in order to make cellular dynamics amenable to investigation by light microscopy. However, it has become clear that many aspects of cell migration depend on features of the local environment including its elasticity, protein composition, and pore size, which are not faithfully represented by rigid two dimensional substrates such as glass and plastic 1. Furthermore, interaction with other cell types, including stromal fibroblasts 2 and immune cells 3, has been shown to play a critical role in promoting the invasion of cancer cells. Investigation at the molecular level has increasingly shown that molecular dynamics, including response to drug treatment, of identical cells are significantly different when compared in vitro and in vivo 4.
Ideally, it would be best to study cell migration in its naturally occurring context in living organisms, however this is not always possible. Intermediate tissue culture systems, such as cell derived matrix, matrigel, organotypic culture (described here) tissue explants, organoids, and xenografts, are therefore important experimental intermediates. These systems approximate certain aspects of an in vivo environment but are more amenable to experimental manipulation such as use of stably transfected cell lines, drug treatment regimes, long term and high-resolution imaging. Such intermediate systems are especially useful as proving grounds to validate probes and establish parameters required to image the dynamic response of cells and fluorescent reporters prior to undertaking imaging in vivo 5. As such, they can serve an important role in reducing the need for experiments on living animals.
Protocol
1. Establishment of fibroblast cultures from skin explants
- 4 mm punch biopsies acquired from human forearm are placed in MEM supplemented 100 units / ml penicillin, 100 μg / ml streptomycin, and 0.25 μg / ml Fungizone.
- Trim any subcutaneous fat and finely chop the biopsy into small pieces by rocking a number 24 scalpel blade against the bottom of the petri dish.
- Place tissue slurry in a 25 cm2 tissue culture flask with primary fibroblast growth media (MEM supplemented with 10% FCS, 100 units / ml penicillin, 100 μg / ml streptomycin, and 25 μg / ml Fungizone) added until the surface of flask is covered but liquid depth is insufficient for tissue to float.
- Following 3 days incubation at 37°C in a humidified atmosphere of 5% CO2 , add 3 ml of primary fibroblast growth media.
- Following another 3 days incubation change media with fresh primary fibroblast growth media, or if cells are nearly confluent they may be split 1:4 into fresh media. (Fungizone can be omitted from this point onwards).
2. Stage I – Preparation of collagen I from rat tails
Note: Protocol for approximately 12-14 adolescent (fresh or frozen) rat tails
- Prepare rat tail by washing in 70% ethanol and remove tendons as follows:
- Remove skin from rat tail by slicing in the middle of the tail from top to bottom with a scalpel and pealing along the length of the tail.
- Detach tendon from the core of the proximal region of the tail.
- Remove tendon toward the distal region of the tail avoiding the sheath using toothed forceps.
- Extract 1 g of tendon / 250 ml of 0.5 M acetic acid by stirring at 4°C for 48 h.
- Centrifuge the extract (7,500 x g) for 30 mins and discard the pellet.
- Add an equal volume of 10% (w/v) NaCl to the supernatant, and stir for 30-60 min.
- Centrifuge (10,000 x g) for 30 mins, discard the supernatant, and re-dissolve the precipitate in 0.25 M acetic acid at ˜ 1:1 ratio by stirring for 24 h at 4°C.
- Dialyze the collagen solution against 6-8 changes of 6L ˜17.5 mM acetic acid (1 ml glacial acetic acid per liter of cold water, change 2 x daily).
- Centrifuge dialysed collagen at 30,000 x g for 1.5 hrs.
- Remove supernatant and place in sterile flask.
- Adjust the collagen I concentration to ˜2 mg/ml using 0.5 mM acetic acid at 4°C.
3. Stage II – Setting up the 3D matrix with embedded fibroblasts (allow to contract for 8 days).
- Assemble the mix using cold reagents at 4°C in a pre-chilled bottle. Keep collagen I on ice.
- For one T75 flask of confluent primary fibroblasts (˜ cell number 1 x 106) use:
- 25 ml rat tail collagen (approx conc 2mg/ml)
- 3 ml 10xMEM
- 0.22 M NaOH: first, add 2ml, then drop-wise whilst stirring, until the collagen turns orange,
- but not pink (usually up to 3 ml). Approximate pH = 7.2.
- Note: It is important to ensure that the medium/gel remains neutral or slightly acidic as fibroblasts will not contract the gel if exposed to even slightly alkaline conditions.
- Trypsinise the fibroblasts, spin at 400 x g for 5 mins and remove supernatant.
- During 5 mins spin above: Prepare 12 x 35 mm plastic dishes – normally achieve 12 dishes from one flask of fibroblasts/collagen mix.
- Re-suspend fibroblasts in 3 ml FCS and immediately add to the collagen mix and stir.
- Plate approximately 2.5 ml of collagen/fibroblast per dish as quickly as possible. Try to avoid bubbles and collagen setting.
- Let the collagen set at 37°C in a humidified atmosphere of 5% CO2 in air for 10 mins.
- Add 1ml of fibroblast growth media (DMEM+ 10% FCS).
- Detach the collagen/fibroblast matrix from the sides of the dish using a pipette.
- Next day: Add 1ml of fibroblast growth media (DMEM + 10% FCS).
- Change media every other day. Allow collagen/fibroblast matrix to contract for approximately 8 days, until they fit in a 24-well dish (Contraction from ˜3.5 cm to 1.5 cm in diameter, see Figure 1).
Note: The collagen concentration should be adjusted to suit the application. The more dilute the collagen solution, the faster it will be contracted by the fibroblasts. Slight differences in contraction rate will be experienced with different batches of collagen at the same concentration. Similarly, different fibroblast cultures will contract the collagen gels at different rates, and the more fibroblasts present, the faster the rate of contraction. Collagen concentration and fibroblast density can therefore by adjusted to modify the rate of contraction, and the final density of the collagen gel.
4. Stage II – Plating cells of interest on top of the matrix
Note: Sterilize all forceps and equipment with ethanol before use.
- Using blunt forceps, gently move contracted matrix to 24-well dish. Make sure it does not fold.
- Prepare suspension of cells of interest at approximately 4 x 104 /ml and plate 1ml (after trypsinising, spin cells to get rid of trypsin) on top of the matrix. Actual number of cells needed will vary depending on cell type used. Cell medium should be normal growth medium for the cells of interest.
- Allow cells to grow to confluence on top of the matrix, approximately 3-5 days.
5. Stage III – Transferring the matrix to a grid for invasion (approximately 0-21 days)
- Cut stainless steel grids to create a tripod and autoclave prior to use (See Figure 2).
- Place sterile grid in 6 cm dish, add growth media to a level above the grid (approx 10.5ml). Place matrix on the grid and gently aspirate media so the bottom of the matrix is in contact with media but not submerged. This is referred to as the air/liquid interface, which creates a gradient that promotes invasion. Replace medium every two days (See Figure 2).
- Live cell, contextual imaging using fluorescent cells can be imaged at this stage or earlier. The contracted or fibrillar collagen I can be imaged using second harmonic generation (SHG, See figure 3). Using multi-photon excitation combined with wide-field detection we find that SHG can be imaged at least 100 μm into the matrix, whereas cells expressing cytoplasmic GFP can be imaged at least 200 μm deep.
Note: With respect to quantification of invasion, the day on which matricies are placed on the grids defines Day 0. Placement on grids generates a gradient of cell culture media that promotes invasion into the matrix. Samples can be imaged over the next 1 – 21 days (or longer) to assess biological processes such as invasion, proliferation, survival or differentiation (See reference list).
6. Stage IV – Fixation
- Add 5 ml of 4% PFA in a Falcon tube.
- Transfer the matrix onto a flat surface. Cut the matrix in half with a fresh clean scalpel (as you will be staining the cross section), lift the matrix with scalpel and transfer to 4% PFA, fix over night.
- Organotypic matrices are now ready to stain with antibody/stain of choice (See Figure 3).
7. Representative results:
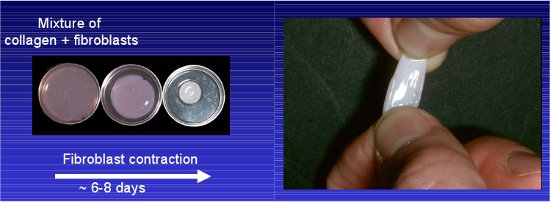
Figure 1 Example of fibroblast-contracted collagen I. Mixture of fibroblast and rat tail collagen detached from the petri dish and allowed to contract over 6-8 days.
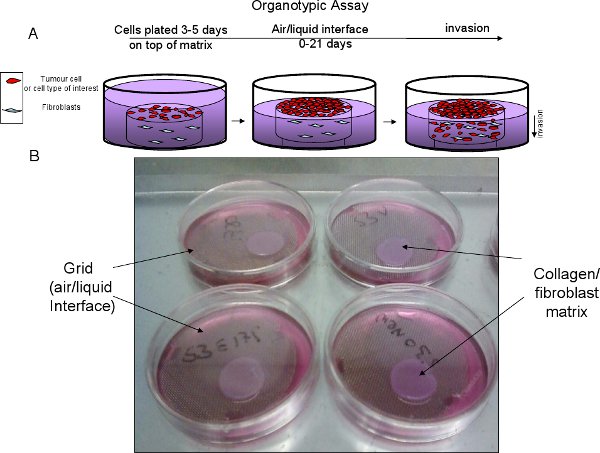
Figure 2 Organotypic assay progression. A, schematic of organotypic set up and progression. B, Example of collagen/fibroblast matrix on top of stainless steel, sterile grid to create air/liquid interface.
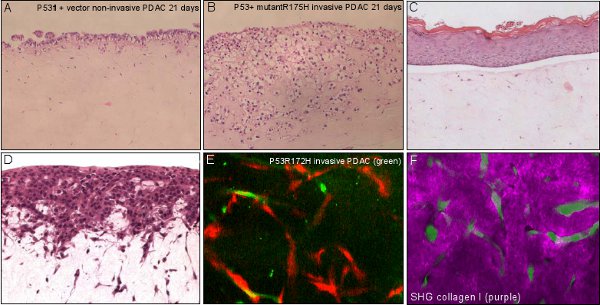
Figure 3 Applications of organotypic assay. A, B non-invasive and invasive pancreatic ductal adenocarcinoma (PDAC) cells invading over time with organotypic matrix. C, living skin equivalent showing a stratified epidermis on a fibroblast-contracted collagenous dermal component. D, C8161 melanoma cells invading in to a fibroblast-contracted collagenous dermal equivalent. E, invasive PDAC cells (green) interacting and invading with fibroblasts (red) within the organotypic matrix. F, invasive PDAC cells (green) interacting with a degrading surrounding extracellular matrix (purple) visualised using multiphoton -based second harmonic generation.
Discussion
Here we present a method for production of a 3-dimensional matrix suitable for studies of invasive cell migration 6-8. The matrix consists of collagen type 1 fibrils, which are contracted over a period of several days by primary human epidermal fibroblasts. The collagen is prepared by acid extraction, not enzymatic digestion, which preserves reactivity at the poly-peptide ends and promotes cross-linking of collagen fibrils into larger aggregates upon neutralization of buffer conditions 9. This results in a re-constituted gel which more closely mimics the features of collagen in vivo and has important consequences for the invasive properties of cells cultured in the gels. It does not matter whether fresh or frozen starting material is used, but use of adolescent rat tails is important because collagen cross-linking is more labile in younger animals. Collagen I from younger animals is therefore easier to extract, and re-constitutes with higher fidelity.
The collagen matrix can be fixed and stained with antibodies directed against either invasive tumor cells or stromal fibroblasts 2,10 and are highly useful for testing the efficacy of drugs which might inhibit invasion 5,6.
Although this protocol suggests the use of primary human skin fibroblasts, it is feasible to use primary fibroblasts from other tissue types, according to the type of tissue under investigation. It is also possible to establish cultures using immortalized fibroblasts, including cells which have been stably transfected with fluorescent protein conjugates. In this way both stromal and tumor cells can be labelled to visualise cell-cell interactions during invasion 11.
Offenlegungen
The authors have nothing to disclose.
Materials
| Name of the reagent | Company | Catalogue number | Comments (optional) |
|---|---|---|---|
| 10x MEM | Gibco | 21430 | – |
| NaOH | Sigma | 367176-500G | Prepare 0.22 M stock in water |
| FCS | PAA Laboratories | A15-101 | – |
| 35 mm dishes | Falcon | 353001 | For step 3.4 |
| 60 mm dishes | Falcon | 353004 | For step 5.2 |
| Spring forceps blunt | Samco | E003/02 | Toothed, not smooth |
| 16% paraformaldehyde | Electron Microscopy Services | 15710 | Dilute to 4% in PBS prior to use |
| Screens for cd-1, size 40 mesh | Sigma | S07707-5EA | Stainless steel grids, 5 per pack |
| Dialysis tubing | Medicell International | 7607 2295 | 12 – 14 kD |
| PBS | Oxoid | BR0014G | – |
| Acetic Acid | Sigma | 242853 | – |
| 24 well dish | Falcon | 353047 | For step 4.1 |
| Fungizone | In vitrogen | 15290018 | – |
Referenzen
- Yamada, K. M., Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell. 130, 601-610 (2007).
- Edward, M., Gillan, C., Micha, D., Tammi, R. H. Tumour regulation of fibroblast hyaluronan expression: a mechanism to facilitate tumour growth and invasion. Carcinogenesis. 26, 1215-1223 (2005).
- Patsialou, A. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res. 69, 9498-9506 (2009).
- Serrels, A. Real-time study of E-cadherin and membrane dynamics in living animals: implications for disease modeling and drug development. Cancer Res. 69, 2714-2719 (2009).
- Timpson, P. Spatial Regulation of RhoA Activity during Pancreatic Cancer Cell Invasion Driven by Mutant p53. Cancer Res. 71, 747-757 (2011).
- Edward, M., Quinn, J. A., Pasonen-Seppanen, S. M., McCann, B. A., Tammi, R. H. 4-Methylumbelliferone inhibits tumour cell growth and the activation of stromal hyaluronan synthesis by melanoma cell-derived factors. Br. J. Dermatol. 162, 1224-1232 (2010).
- Fusenig, N. E. Growth and differentiation characteristics of transformed keratinocytes from mouse and human skin in vitro and in vivo. J. Invest. Dermatol. 81, 168s-175s (1983).
- Nystrom, M. L. Development of a quantitative method to analyse tumour cell invasion in organotypic culture. J. Pathol. 205, 468-475 (2005).
- Sabeh, F., Shimizu-Hirota, R., Weiss, S. J. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J. Cell. Biol. 185, 11-19 (2009).
- Amjad, S. B., Carachi, R., Edward, M. Keratinocyte regulation of TGF-beta and connective tissue growth factor expression: a role in suppression of scar tissue formation. Wound Repair Regen. 15, 748-755 (2007).
- Gaggioli, C. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell. Biol. 9, 1392-1400 (2007).

