Preparing Undercut Model of Posttraumatic Epileptogenesis in Rodents
Summary
Partially isolated cortex (“undercut”) is an efficient animal model of posttraumatic epileptogenesis. Here we demonstrate how to make a novel surgical device and use it to make more precise and consistent lesions to generate this model.
Abstract
Partially isolated cortex (“undercut”) is an animal model of posttraumatic epileptogenesis. The surgical procedure involves cutting through the sensorimotor cortex and the underneath white matter (undercut) so that a specific region of the cerebral cortex is largely isolated from the neighboring cortex and subcortical regions1-3. After a latency of two or more weeks following the surgery, epileptiform discharges can be recorded in brain slices from rodents1; and electrical or behavior seizures can be observed in vivo from other species such as cat and monkey4-6. This well established animal model is efficient to generate and mimics several important characteristics of traumatic brain injury. However, it is technically challenging attempting to make precise cortical lesions in the small rodent brain with a free hand. Based on the procedure initially established in Dr. David Prince’s lab at the Stanford University1, here we present an improved technique to perform a surgery for the preparation of this model in mice and rats. We demonstrate how to make a simple surgical device and use it to gain a better control of cutting depth and angle to generate more precise and consistent results. The device is easy to make, and the procedure is quick to learn. The generation of this animal model provides an efficient system for study on the mechanisms of posttraumatic epileptogenesis.
Protocol
1. Making a simple device for undercut surgery
- The undercut device we created consists of three parts (Fig. 1): (1) a supporting plate made of stainless steel or plastic that allows attachment of a guiding tube and a needle, and sits across the cranial window during surgery; (2) a guiding tube that holds a needle in position and permits needle rotation; and (3) a needle that is bent 90 degrees at ~3 mm from the tip, and is movable by rotation and insertion.
- Cut a piece of 1-1.5 mm thick, 7~10 x 30 mm rectangular stainless steel or transparent plastic (1) to make the supporting plate. Prepare a 1.5 inch 22-gauge (BD company, #305156) and a 1.5 inch 25-gauge (BD company, #305127) syringe needle. To make a guiding tube, cut off the plastic end and the lower needle tip of the 22-gauge syringe needle so that its total length is about 31-32 mm (2). Sand the metal end to make it flat and smooth. The final length of this guiding tube should be about 5-6 mm shorter than the 25-gauge syringe needle.
- Use cyanoacrylate glue to fix the guiding tube onto the supporting plate, making sure that the needle is perpendicular to the edge of the metal.
- Insert the 25-gauge syringe needle into the guiding tube, and bend the needle 90 degrees at 2.5-3 mm from the tip (Fig. 1).
- Adjust the vertical moving range of the needle by gluing a small plastic tube onto the up end of the needle (Fig. 1, needle stop). The final moving range of the needle determines how deep the needle can be inserted into the cortex, and should be 1.6-1.8 mm for P21 rats and 1.3-1.5 mm for P21 mice. This length needs to be adjusted for different species and ages of animals.
- Attach a small copper wire or a small piece of tape onto the upper end of the needle in the same direction as the bent tip (Fig.1 needle indicator). The wire or tape indicates the turning angle of the needle during undercut surgery (Fig. 1).
2. Animal preparation
- All surgical instruments need to be autoclaved, sterilized with a glass-bead sterilizer, or disinfected with 70% ethanol.
- We use Sprague/Dawley rats at postnatal age 20 – 22 days (P20-22) or CD1 mice at the same age. Different strains of mice or rats may be used for specific projects.
- Anesthetize the mouse with an i.p injection of ketamine 80 mg/kg and xylazine 8 mg/kg. The surgical procedure starts after the animal does not respond to tail pinch. During the surgery, if necessary, a booster dose of one-third the original dose of the anesthetic cocktail can be given to restore the original anesthetic state.
- Cut the hair on the scalp of the animal with an electrical hair trimmer. Apply a small amount of ophthalmic ointment onto the eyes of the animal for protection from dryness during anesthesia. Disinfect the scalp using a 10% povidone-iodine solution, followed by 70% ethanol.
- Mount the animal onto a stereotaxic apparatus to keep the head in a stable fixed position. Throughout the surgery, we keep the animal on a heating pad to prevent hypothermia.
- Make a midline anterior-posterior incision on the scalp using a scalpel, extending from lambda to between the eyes. Use hemostats to pull the skin aside and expose the left skull sufficiently.
- Make a rectangular cut on the left skull, and use the scalpel to scrape off periosteum. This step will reduce bleeding and facilitate drilling on the skull.
3. Making undercuts
- Add a small amount of sterile saline on to the exposed skull area, and then use several Q-tips to clean blood and dry the area.
- Under a surgical microscope, start drilling a rectangular groove (~5 x 7 mm in rats, 4 x 5 mm in mice) on the center of the left skull (approximately above the left sensorimotor cortex). Applying a drop of saline on the skull facilitates drilling and dissipates heat. After approximately 2/3 depth of the bone is drilled, remove excess saline and clean the drilling area with a Q-tip. Slowly and carefully drill deeper until the center piece of bone is moveable upon gentle touch of a forceps.
- Carefully remove the central piece of bone by inserting a sharp tip of a forceps onto the edge of the bone and slowly lifting the forceps to expose the left hemisphere.
- Under the surgical microscope, hold the undercut device, and orient the needle in a parasagittal direction and the supporting plate perpendicular to the midline, tilting the device slightly caudally so as to maintain visualization of the needle tip and target cortical region. Aim the tip of the needle to an area 1-2 mm lateral to the superior sagittal suture, in the middle of the cranial window, and avoid direct penetration of large vessels.
- Insert the needle in a horizontal direction through the dura and under the pia so as to spare blood vessels. Sit the bottom of the undercut device onto both edges of the cranial window so that the needle is positioned normal to the cortical surface (Fig. 1). Resting the device on the both edges of cranial window by hand will significantly reduce or eliminate hand shaking during the following procedure. Lift up the needle to underneath the pia, then slowly lower it to create a transcortical cut until the needle cannot go deeper. Rotate ~135° away from the midline to create a half-circle white matter/deep layer VI undercut. Then raise the needle again to underneath the pia. Tilt the device backward and withdraw the needle.
- Optionally, one can repeat the above procedure (step 2.5) but without turning the needle so as to create an additional transcortical cut at the lateral edges of the window. This will create a more complete cortical isolation.
- Place a piece of plastic film (6 x 6 mm) onto the cranial window for protection, and suture the scalp. Place the animal on a heated pad until fully recovered from anesthesia.
4. Representative results:
Coronal cortical slices can be prepared to confirm the success of the undercut surgery. In slices prepared >2 weeks after surgery, transcortical and undercut cuts are discernible under low power objective of a microscope (Fig. 2). The partially isolated cortex usually becomes slightly thinner, and epileptiform activity can be detected in majority of cortical slices with field potential recording (Fig. 3).
In contrast, formation of big holes in the white matter or in deep cortical layers, dramatic thinning of the lesioned cortex, or an errant cut being above or below white matter will make the brain unusable for further experiments.
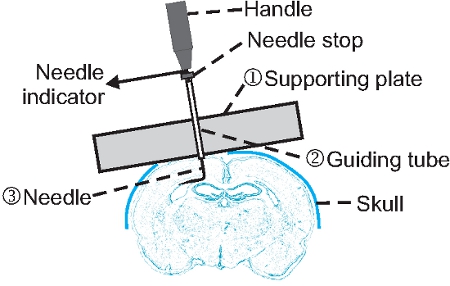
Figure 1. Structure and application of an undercut device. A guiding tube (2) is glued onto a supporting plate (1) that is made of stainless steel or transparent plastic. A syringe needle (3) is inserted through the guiding tube and bent at ~3 mm to the tip. A needle stop made of plastic tube is glued onto the upper end of the needle so that the vertical moving range of the needle is limited to ~ 1.2 mm and ~1.5 mm for use in P21 mice and rats respectively. A segment of small copper wire is fastened beneath the handle as needle indicator for the orientation of the bent needle. Note that the device is tilted and in contact with both edge of the cranial window so that a cut can be made parallel to the pial surface.
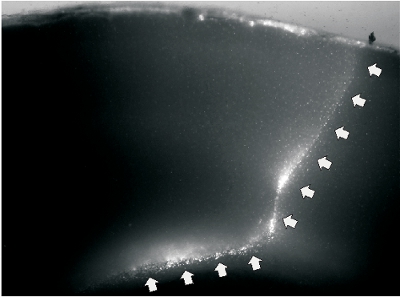
Figure 2. A representative image of undercut slice. A. Fluorescent image of a slice that was prepared two weeks after undercut lesion in a P48 rat. The cutting wound was labeled by fluorescent dye DiI. The white arrows on the right indicate transcortical cut, and the arrows on the bottom indicate undercut that passed though the border between layer IV and white matter.
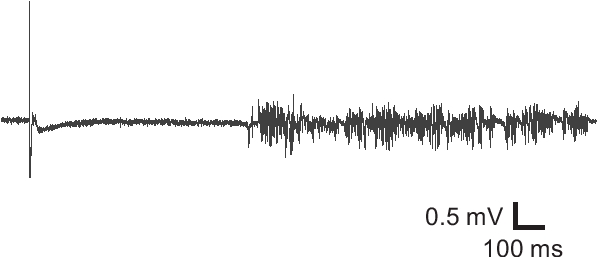
Figure 3. Field potential recording from an undercut slice. Field potential recording from an undercut brain slice exhibited epileptiform activity, suggesting hyperexcitability of the injured cortical tissue.
Discussion
The undercut model is a highly efficient system for studying posttraumatic epileptogenesis. A typical surgery takes only about 20-30 minutes to finish, and evoked or spontaneous epileptiform activity can be recorded in slices from most animals two weeks after surgery1-2. More importantly, this model mimics aspects of changes following traumatic brain injury such as bleeding, inflammation, edema, axotomy and neuronal death7. Not only has epileptiform activity been observed in rodents and other animals, but also epileptic seizures have been documented in humans who suffer comparable cortical lesions8. Significant progress has been made to elucidate the underlying mechanisms in recent years. Spontaneous and evoked interictal epileptiform discharges have been recorded in brain slices >2 weeks after the lesion, and these activities were found to originate in rat cortical layer V1. Evidence of circuit reorganization, loss of GABAergic interneurons and disinhibition, increases in neuronal membrane excitability, and increases in excitatory synaptic coupling have also been demonstrated, particularly in cortical layer V 2,9-13.
Here we introduced a novel instrument for making undercut lesions. When one performs undercut surgery with a free hand, hand shaking frequently causes difficulty in making stable and precise cortical lesions. Although practice and experience may improve the quality of the surgery, significant variability in the depth and quality of the lesions exists. The device we have introduced here is beneficial in three aspects. First, by resting on the edges of the cranial window, the device largely eliminates the problem of hand shaking during the surgery, thus making it possible to make a smooth cut through the delicate brain tissue. Second, the depth of the needle insertion and the degree of rotation can be carefully controlled, which makes it possible to cut more precisely and consistently in the white matter beneath layer VI. Third, the surface of the cortical hemisphere is curved: with the medial being higher than the lateral side (Fig. 1), which can cause missing the targeted white matter if the angle of the needle is not adjusted accordingly. By resting the device on the cranial window, the needle is tilted laterally, and the cutting angle is automatically adjusted so that the rotation of the needle is always parallel to the surface of the cortex and precise lesion is obtained (Fig. 2). One potential problem with using this device is that it may interfere with direct visualization of the needle under the microscope. This problem can be resolved by slightly tilting the device toward the caudal direction when penetrating the pial and the cortex. As soon as the needle is lowered to the white matter, the device needs to be adjusted to become vertical to the cortical surface, and rested on the skull. At this point, looking at the needle indicator is sufficient to monitor needle rotation. In summary, with these several advantages and its relative ease of construction, the undercut model will become more accessible and useful for the study of posttraumatic epileptogenesis.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH/NINDS grant 4R00 NS 057940, and grant SCBI 200-12 from the Spinal Cord and Brain Injury Research fund from the Indiana State Department of Health.
Materials
| Material Name | Typ | Company | Catalogue Number |
| Foredom micromotor kit | equipment | Foredom | K.1070 |
| 1.5 inch 22-gauge syringe needle | material | BD company | 305156 |
| 1.5 inch 25-gauge syringe needle | material | BD company | 305127 |
| Cyanoacrylate glue | material | Ted Pella | 14450 |
Referenzen
- Hoffman, S. N., Salin, P. A., Prince, D. A. Chronic neocortical epileptogenesis in vitro. J Neurophysiol. 71, 1762-1773 (1994).
- Topolnik, L., Steriade, M., Timofeev, I. Hyperexcitability of intact neurons underlies acute development of trauma-related electrographic seizures in cats in vivo. Eur J Neurosci. 18, 486-496 (2003).
- Graber, K., Prince, D. A. . Models of Seizures and Epilepsy. , 477-493 (2005).
- Nita, D. A., Cisse, Y., Timofeev, I., Steriade, M. Increased propensity to seizures after chronic cortical deafferentation in vivo. J Neurophysiol. 95, 902-913 (2006).
- Sharpless, S. K., Halpern, L. M. The electrical excitability of chronically isolated cortex studied by means of permanently implanted electrodes. Electroencephalogr Clin Neurophysiol. 14, 244-255 (1962).
- Echlin, F. A., Battista, A. Epileptiform Seizures from Chronic Isolated Cortex. Arch Neurol. 9, 154-170 (1963).
- Prince, D. A. Epileptogenic neurons and circuits. Adv Neurol. 79, 665-684 (1999).
- Marin-Padilla, M. Developmental neuropathology and impact of perinatal brain damage. II: white matter lesions of the neocortex. J Neuropathol Exp Neurol. 56, 219-235 (1997).
- Jin, X., Prince, D. A., Huguenard, J. R. Enhanced excitatory synaptic connectivity in layer v pyramidal neurons of chronically injured epileptogenic neocortex in rats. J Neurosci. 26, 4891-4900 (2006).
- Li, H., Prince, D. A. Synaptic activity in chronically injured, epileptogenic sensory-motor neocortex. J Neurophysiol. 88, 2-12 (2002).
- Salin, P., Tseng, G. F., Hoffman, S., Parada, I., Prince, D. A. Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci. 15, 8234-8245 (1995).
- Avramescu, S., Nita, D. A., Timofeev, I. Neocortical post-traumatic epileptogenesis is associated with loss of GABAergic neurons. J Neurotrauma. 26, 799-812 (2009).
- Avramescu, S., Timofeev, I. Synaptic strength modulation after cortical trauma: a role in epileptogenesis. J Neurosci. 28, 6760-6772 (2008).

