Assessing Burrowing, Nest Construction, and Hoarding in Mice
Summary
Burrowing, nesting, and hoarding are species-typical activities that mice readily perform in the laboratory. This article describes how they can be easily and cheaply assessed. These protocols are extremely sensitive to mouse strain, brain lesions and diseases. Moreover they constitute “environmental enrichment” for the mice, and embody the “Refinement” aspect of the “3 Rs”18.
Abstract
Deterioration in the ability to perform “Activities of daily living” (ADL) is an early sign of Alzheimer’s disease (AD). Preclinical behavioural screening of possible treatments for AD currently largely focuses on cognitive testing, which frequently demands expensive equipment and lots of experimenter time. However, human episodic memory (the most severely affected aspect of memory in AD) is different to rodent memory, which seems to be largely non-episodic. Therefore the present ways of screening for new AD treatments for AD in rodents are intrinsically unlikely to succeed. A new approach to preclinical screening would be to characterise the ADL of mice. Fortuitously, several such assays have recently been developed at Oxford, and here the three most sensitive and well-characterised are presented.
Burrowing was first developed in Oxford13. It evolved from a need to develop a mouse hoarding paradigm. Most published rodent hoarding paradigms required a distant food source to be linked to the home cage by a connecting passage. This would involve modifying the home cage as well as making a mouse-proof connecting passage and food source. So it was considered whether it would be possible to put the food source inside the cage. It was found that if a container was placed on the floor it was emptied by the next morning., The food pellets were, however, simply deposited in a heap at the container entrance, rather than placed in a discrete place away from the container, as might be expected if the mice were truly hoarding them. Close inspection showed that the mice were performing digging (“burrowing”) movements, not carrying the pellets in their mouths to a selected place as they would if truly hoarding them.6
Food pellets are not an essential substrate for burrowing; mice will empty tubes filled with sand, gravel, even soiled bedding from their own cage. Moreover, they will empty a full tube even if an empty one is placed next to it8.
Several nesting protocols exist in the literature. The present Oxford one simplifies the procedure and has a well-defined scoring system for nest quality5.
A hoarding paradigm was later developed in which the mice, rather than hoarding back to the real home cage, were adapted to living in the “home base” of a hoarding apparatus. This home base was connected to a tube made of wire mesh, the distal end of which contained the food source. This arrangement proved to yield good hoarding behaviour, as long as the mice were adapted to living in the “home base” during the day and only allowed to enter the hoarding tube at night.
Protocol
1. Burrowing
1. Apparatus
Various container designs were tried (for example a metal jug that could be easily cleaned, but mice were reluctant to enter it). Eventually it was discovered that a length of plastic downpipe (as connected to guttering on houses) elevated slightly at one end to prevent accidental (non-deliberate) displacement of food pellets and sealed at the other end, was an efficient piece of apparatus. Burrows are now made from plastic downpipe, 68 mm diameter, cut into 20 cm long lengths (Figure 1). One end is sealed with a plug of 12 mm medium density fibreboard (mdf). (Respiratory protection is advised when working with mdf). For cleaning purposes and waterproofing, a waterproof adhesive mastic compound can be applied to the inside and outside surfaces of the mdf as well as serving to glue it into the burrowing tube. Machine screws (5 cm long) are used to elevate the other end of the tube 3 cm off the floor. Two holes are drilled 1 cm from the open end of the burrowing tube at an angle of 90° and the screws inserted and tightened4.
The diameter of the burrows is probably critical; Schmid-Holmes et al.19 observed that mice in the wild ‘burrow cleaned’ mainly from burrows similar in size to our artificial ones.

Figure 1. A mouse in a burrowing tube.
2. Procedure
- Habituation
Burrowing is normally spontaneous, but there is a certain learning element. The learning process is enhanced by social facilitation. Therefore place a full burrow overnight into the group home cage of mice which are later to be tested with a treatment. Each burrow is filled with 200 g of the food pellets normally supplied as diet. (This refers to the dense compressed type of diet; if an ‘expanded’ diet is in use the burrow will hold less than 200g). Experimenters should take into consideration such factors when selecting a burrowing substrate.
If the mice are on food rationing (for example, to motivate them to run a concurrent maze learning task) non-food substances can be used, for example clay balls, as used to line the surface of the soil in indoor plant pots; pea shingle (small stones ~1 cm diameter) and sand. Pea shingle and sand, being three times heavier than food pellets, are useful substitutes for food pellets if the mice are burrowing very vigorously, to avoid a ‘ceiling effect’. - Baselining
Run (preferably two) baseline burrowing tests 48 h apart in the same way as will be done for testing after the proposed treatment. The test is optimally started around 4 pm if the mice are housed on an 0700-1900 h dark-light cycle. This is a sensitive time to test, as they are not normally awake at this time and burrowing starts more slowly than if they were well in to the dark phase and consequently very active. Two hours after the start, a ‘snapshot’ measurement is taken of the weight of food burrowed; the burrow is emptied into a tared container and weighed and the pellets replaced into the burrow which is put back into the cage. If the mouse is in the burrow, just gently pour it and the contents into the weighing container and replace the mouse in the cage. The weight burrowed at two hours is then calculated by subtraction from the original 200 g. The final reading is taken the next morning. Timing is not critical as the mice will have gone back to sleep (often in the burrow) and are no longer actively burrowing. Use the 2 h measurement taken on the second trial to form groups balanced for baseline performance. As burrowing increases with practice17, baselining also helps the mice to reach more stable asymptotic performance which reveals treatment effects better. - Testing
For testing, after treatments are administered the mice are given the burrowing test exactly the same as in the baselining stage. If the burrowing test is to be used chronically, it is recommended that at least 48 h separate the tests to prevent a decline in performance due to over-exposure to the procedure. - Note
Do NOT replace the burrowed food on the cage floor into the burrow. Even if the mouse has not burrowed anything, perform the weighing procedure as you would if burrowing had occurred; the disturbance caused by weighing tends to stimulate behaviour, and mice which have already burrowed some of the substrate will often commence burrowing with renewed vigour after the weighing process.
2. Expected results
C57~BL/6 mice typically burrow around 70 g in the first two hours, and near 200 g overnight. Hippocampal lesioned mice often burrow less than 5 g11 (Figure 2). Prion disease inhibits burrowing1,2 , as does lipopolysaccharide administration20. Burrowing can detect prion (scrapie) disease at 10-12 weeks after injection of diseased brain homogenate, whereas clinical signs only appear at 22 weeks13. (Figure 3). Burrowing has been shown to be sensitive to strain differences15 and knock-out of potassium ion channel subunits10.
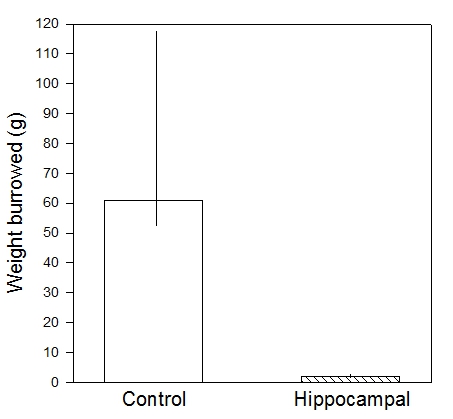
Figure 2. Weight of food pellets (medians and interquartile ranges) burrowed in two hours by control and hippocampal-lesioned mice. The latter burrowed significantly less than controls (P = 0.0001).
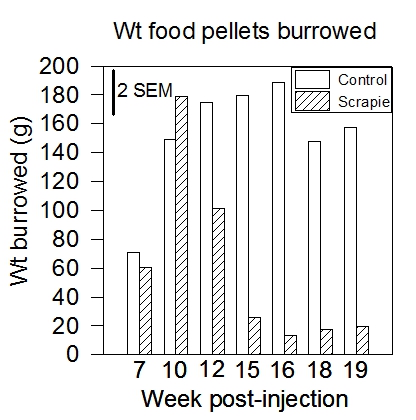
Figure 3. Weight of food pellets (means) burrowed overnight by control and scrapie-infected mice. Burrowing increases (with practice) in both groups from week 7 to 10 post-injection, but from week 12 burrowing declines in the scrapie group and the difference between the groups becomes statistically significant (exceeds two standard errors of the mean).
3. Discussion
We have tested burrowing in other species of rodents – rats, gerbils, hamsters and Egyptian spiny mice8. All but the latter could be induced to burrow substantial amounts of terrestrial substrates such as gravel or sand, but burrowing of food pellets was much less, even after extensive experience with the terrestrial materials. The reason for this difference from mice is not known. After this work had been done, it was discovered that Egyptian spiny mice do not in fact make burrows in the wild; they may occupy burrows made by gerbils, also the surface of their natural habitat is often hard rock. Recent observation in Nairobi showed that, within the genus Acomys, Wilson’s spiny mouse (Acomys wilsoni) and Percival’s spiny mouse (Acomys percivali), which do burrow in the wild, also burrowed in the laboratory.
4. Nesting
1. Apparatus
This test is performed in individual cages; the same ones as used for burrowing are suitable. Normal bedding should cover the floor to a depth of 0.5 cm. (Variation in depth, and very deep bedding, could affect nest construction). Each cage is supplied with a ‘Nestlet’, a 5 cm square of pressed cotton batting (Ancare).
2. Procedure
Mice are placed individually into the nesting cages about one hour before the dark phase, and the results are assessed the next morning. As with burrowing, and for similar reasons, timing is not critical.
3. Scoring
The nests are assessed on a 5-point scale (Figures 4-8), and the amount of untorn Nestlet is also weighed5.
- The Nestlet is largely untouched (>90% intact).
- The Nestlet is partially torn up (50-90% remaining intact).
- The Nestlet is mostly shredded but often there is no identifiable nest site: < 50% of the Nestlet remains intact but < 90% is within a quarter of the cage floor area, i.e. the cotton is not gathered into a nest but spread around the cage. Note: the material may sometimes be in a broadly defined nest area but the critical definition is that 50-90% has been shredded.
- An identifiable, but flat nest: > 90% of the Nestlet is torn up, the material is gathered into a nest within a quarter of the cage floor area, but the nest is flat, with walls higher than mouse body height (curled up on its side) on less than 50% of its circumference.
- A (near) perfect nest: > 90% of the Nestlet is torn up, the nest is a crater, with walls higher than mouse body height on more than 50% of its circumference.
Where criteria do not agree split the difference. For example a perfect nest with an unshredded 0.7g piece would score 4.5.

Figure 4. Nest score 1

Figure 5. Nest score 2

Figure 6. Nest score 3

Figure 7. Nest score 4

Figure 8. Nest score 5
4. Expected results
Both males and females make nests, as their purpose is thermoregulation as well as being associated with reproduction. For C57BL/6 mice, nest scores for males and females are in the same range, although we have not done a controlled comparison in a single experiment. Most C57BL/6 mice score 4-5 on nest construction, but when the hippocampus is lesioned the median score would be around 1-2; a score of 3 is unlikely to be exceeded (Figure 9). Nesting also proved to be sensitive to prion disease around 10-12 weeks1,2,16.
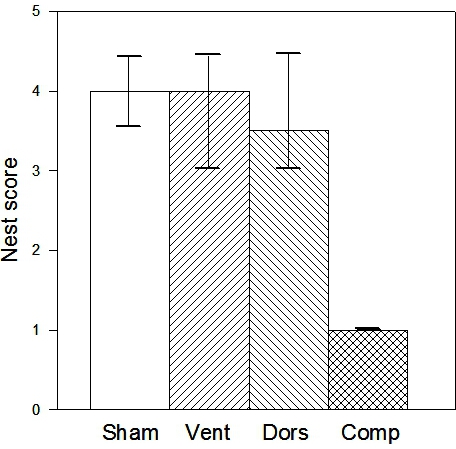
Figure 9. Nest scores (medians and interquartile ranges) for control mice and mice with lesions of the dorsal, ventral and complete hippocampus. Only the complete lesioned mice show inhibition of nesting, so the effects of selective dorsal and ventral regions appear sub-threshold but additive.
5. Hoarding
1. Apparatus
A series of ‘home bases’ are connected to tubes made of wire mesh, sealed permanently at the distal end where the food pellets are placed and temporarily at the proximal end by a wooden plug. This is used to prevent the mice entering the tubes before adaptation to the home base has occurred7.
The apparatus (Figure 10) can be made in various ways, as long as the basic principles are adhered to. Ours consists of a row of 8 wooden boxes, each 30 x 13 x 15 cm with transparent Perspex lids. Each is supplied with a water bottle, and has a hole in the back into which the hoarding tube is push-fitted. Tubes are made of black plastic, 10 cm long, 40 mm diameter, joined to a wire mesh tube, 45 cm long, 4 cm diameter, to form a total length of 50 cm. The mesh consists of 13 mm squares, and is a double roll with the meshes mis-aligned to make the holes in the mesh smaller and prevent food pellets dropping through. If 6 mm square mesh is available this is a better material as one roll would be sufficient. The distal end of the tube, where the food pellets are placed, is closed. The proximal end is sealed with a removable wooden plug.

Figure 10. Mice in the hoarding tubes
2. Procedure
- Typically, hoarding is very variable the first time and some mice may not hoard at all. Hoarding, like burrowing, improves with practise. Therefore, where experimental design allows, baselining sessions are best given before a treatment is administered. Clearly, this is not practical if the independent variable is innate, as with a (non-conditional) mutant mouse or when testing different mouse strains.
- A mouse is put into each box early in the day to habituate to it. The box is provided with a cardboard tunnel which has been in the real group home cage for at least one night, also a generous portion of soiled bedding, to make it feel like ‘home’. Access to the hoarding tube is prevented until evening with a wooden bung. 100 g food pellets (a mixture of small and large) is placed at the distal end of the hoarding tube (by pouring them in at the other end with the tube held vertically). To make sure the mouse is mildly hungry before testing begins, the home box is not provided with food.
- Just before the start of the dark phase, remove the wooden bung to allow the mouse access to the hoarding tube. The next morning (again, timing is not critical) collect and weigh all the food pellets which have been hoarded into the home base box.
6. Expected results
Using female C57BL/6 mice, the median amount hoarded would be around 50-70 g. Hippocampal lesions strongly suppress hoarding11, whereas prefrontal cortex lesions have only a weak effect12 (Figure 11).
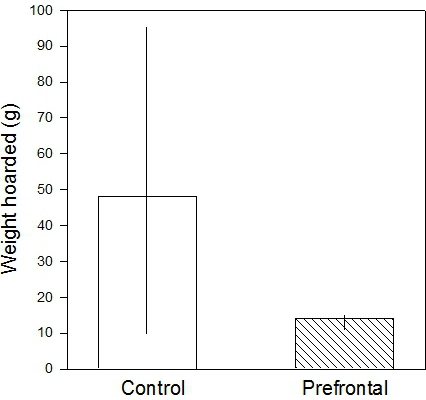
Figure 11. Weight of food pellets hoarded by control and medial prefrontal cortex-lesioned mice (medians and interquartile ranges). Although the medians differ considerably, the high variability in the controls means the result is far from statistically significant (P = 0.3).
Discussion
These tests are extremely simple to run but the effect sizes are very large, making them extremely sensitive to treatments, strain differences, etc. They conform to the ‘Refinement’ aspect of Russell and Burch’s 3 Rs’18. Indeed, good performance on tests like burrowing seems to be indicative of good general health in animals. Therefore this could be a good test for assessment of animal welfare.
The importance of environmental enrichment for laboratory rodents is well recognised these days. Provision of nesting material is now standard practice in many animal houses, and this is easy to do. The enthusiasm with which mice burrow makes burrowing a potentially excellent enrichment, but unfortunately a ‘self-reloading’ burrow which would be a practical way of providing this has not yet been developed. Whether mice find hoarding enriching has not yet been determined.
One drawback is that the data generated by these tests is generally non-parametric (not Gaussian in distribution), so appropriate non-parametric statistics are needed to analyse them, e.g. the Mann-Whitney U test for pairwise comparisons, Kruskal-Wallis ANOVA for multi-group data. If repeated measures designs are used, since there is no suitable ANOVA test for non-parametric data, the results can be transformed, e.g. by square root or log procedures to make them conform to a more normal distribution.
The measurements of the apparatus can be slightly different to those specified in the text (useful for USA experimenters) but wide differences should be avoided. However, in some cases other experimenters may find different constructions work better than those described here.
As for all behavioural measurements, the mice should be tested to see if their activity levels are normal, as inactivity will obviously have a detrimental effect on species-typical behaviours. When scrapie-infected mice were showing impaired burrowing and nesting, their activity levels were normal (tested in an open field apparatus). Indeed, a period of hyperactivity occurs around 2-4 weeks after these species-typical behaviours are first impaired2.
The strong impairment produced by hippocampal lesions on burrowing, nesting and hoarding, all of which are species-typical behaviours, complements the impairments in spatial learning and memory9 which are such well-established effects of these lesions. They appear to be equivalent to the impairments in the activities of daily living so characteristic of AD14.
These species-typical tests may well prove to be of great use in preclinical screening for treatments for Alzheimer’s and other neurodegenerative diseases14. In many human brain disorders a loss or reduced ability to perform normal everyday tasks is as important as other features of the disease like cognitive impairment. Particularly, this loss may necessitate involvement of carers and the great socioeconomic burden this so often imposes.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The Wellcome Trust for providing Open Access funding to Oxford University. Robert Deacon is a member of Oxford OXION group, funded by Wellcome Trust grant WT084655MA.
Referenzen
- Cunningham, C., Deacon, R. M. J., Chan, K., Boche, D., Rawlins, J. N. P., Perry, V. H. Neuropathologically distinct prion strains give rise to similar temporal profiles ofbehavioural deficits. Neurobiol. Dis. 18, 258-269 (2005).
- Cunningham, C., Deacon, R., Wells, H., Boche, D., Waters, S., Picanco Diniz, C., Scott, H., Rawlins, J. N. P., Perry, V. H. Synaptic changes characterize early behavioural signs in the ME7 model of murine prion disease. Eur. J. Neurosci. 17, 2147-2215 (2003).
- Damiani, R., Modesto, S., Yates, A., Neveling, J. Earliest evidence of cynodont burrowing. Proc. R. Soc. Lond. B. 270, 1747-1751 (2003).
- Deacon, R. M. J. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nat. Protocols. 1, 118-121 (2006).
- Deacon, R. M. J. Assessing nest building in mice. Nat. Protocols. 1, 1117-1119 (2006).
- Deacon, R. M. J., Gould, T. D. Digging in Mice: Marble Burying, Burrowing, and Direct Observation Reveal Changes in Mouse Behavior. Mood and anxiety related phenotypes in mice. , (2009).
- Deacon, R. M. J. Assessing hoarding in mice. Nat. Protocols. 1, 2828-2830 (2006).
- Deacon, R. M. J. Burrowing: A sensitive behavioural assay, tested in five species of laboratory rodents. Behav. Brain. Res. 200, 128-133 (2009).
- Deacon, R. M. J., Bannerman, D. M., Kirby, B. P., Croucher, A., Rawlins, J. N. P. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav. Brain. Res. 133, 57-68 (2002).
- Deacon, R. M. J., Brook, R. C., Meyer, D., Haeckel, O., Ashcroft, F. M., Miki, T., Seino, S., Liss, B. Behavioral phenotyping of mice lacking the KATP channel subunit Kir6.2. Physiol. Behav. 87, 723-733 (2006).
- Deacon, R. M. J., Croucher, A., Rawlins, J. N. P. Hippocampal cytotoxic lesion effects on species-typical behaviors in mice. Behav. Brain. Res. 132, 203-213 (2002).
- Deacon, R. M. J., Penny, C., Rawlins, J. N. P. Effects of medial prefrontal cortex cytotoxic lesions in mice. Behav. Brain. Res. 139, 139-155 (2003).
- Deacon, R. M. J., Raley, J. M., Perry, V. H., Rawlins, J. N. P. Burrowing into prion disease. Neuroreport. 12, 2053-2057 (2001).
- Deacon, R. M. J., Rawlins, J. N. P. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behav. Brain. Res. 156, 241-249 (2005).
- Deacon, R. M. J., Thomas, C. L., Rawlins, J. N. P., Morley, B. J. A comparison of the behavior of C57BL/6 and C57BL/10. Behav. Brain. Res. 179, 239-247 (2007).
- Guenther, K., Deacon, R. M. J., Perry, V. H., Rawlins, J. N. P. Early behavioural changes in scrapie-affected mice and the influence of dapsone. Eur. J. Neurosci. 14, 401-409 (2001).
- Mallucci, G. R., White, F. a. r. m. e. r., Dickinson, M., Khatun, A., Powell, H., Brandner, A. D., Jefferys, S., R, J. G., Collinge, J. Targeting Cellular Prion Protein Reverses Early Cognitive Deficits and Neurophysiological Dysfunction in Prion-Infected Mice. Neuron. 53, 325-335 (2007).
- Russell, W. M. S., Burch, R. L. . The principles of humane experimental technique. , (1959).
- Schmid-Holmes, S., Drickamer, L. C., Robinson, A. S., Gillie, L. L. Burrows and burrow cleaning behaviour of house mice. Am. Mid. Nat. 146, 53-62 (2001).
- Teeling, J. L., Felton, L. M., Deacon, R. M. J., Cunningham, C., Rawlins, J. N. P., Perry, V. H. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav. Immun. 21, 836-850 (2007).

