External Excitation of One-Dimensional Patterned Neuronal Cultures
Abstract
Source:Stern, S., et al. External Excitation of Neurons Using Electric and Magnetic Fields in One- and Two-dimensional Cultures. J. Vis. Exp. (2017)
This video demonstrates a technique for stimulating line-patterned neuronal cultures with a uniform, unidirectional electric field.
Protocol
1. Imaging of spontaneous or evoked activity in neuronal cultures with fluorescent dyes.
- Prepare a solution of 50 µg calcium sensitive fluorescent dye (see Table of Materials/Reagents) in 50 µL DMSO (dimethyl sulfoxide).
- Prepare extracellular recording solution (EM) containing (in mM) 10 HEPES, 4 KCl, 2 CaCl2, 1 MgCl2, 139 NaCl, 10 D-glucose, 45 sucrose (pH 7.4).
- Incubate neuronal culture in 2 mL EM with 8 µL of the calcium-sensitive fluorescent dye solution for 1 h. Protect from light and gently rotate to ensure a homogenous spread of the dye to the cells.
- Replace the solution with fresh EM prior to imaging. The fluorescent imaging is demonstrated in Figure 1.
- Image in a fluorescence microscope with optical filters for calcium fluorescence imaging (excitation peak at 488 nm, emission peak at 520 nm), using a camera and software capable of quantifying the intensity of any region of interest (ROI) within the field of view of the microscope.
2. Electric Stimulation of Cultures
NOTE: The basic setup for electric stimulation is shown in Figure 2. A cover slip on which the neuronal culture has been grown for about 14 days is placed in a Petri dish under a fluorescence microscope. Electrical activity of the neurons is imaged using calcium sensitive dyes while a voltage is applied via two pairs of bath electrodes that are positioned outside the culture. The electrodes are driven by a signal generator whose output is amplified by a dual channel amplifier. Voltage control for stimulation is preferred over the more standard current control, because the electric field vectors are determined directly, thus enabling straightforward vector addition and combination. This does require a careful check of the uniformity of the electric field, which can be performed over the whole sample for the case of voltage control. When using voltage control care should be taken to avoid any ground loops and the homogeneity of the electric field should be verified.
- For electric stimulation with a homogeneous electric field use a pair of parallel electrode wires.
- Use electrodes made of platinum with a thickness on the order of 0.005'' (127 µm). When used with the 13 mm coverslips, ensure that the distance between the two electrodes is around 11 mm, and position the electrodes 1 mm above the culture.
NOTE: To make the electrode holder (Figures 3A), use polytetrafluoroethylene (PTFE). Drill narrow holes through the PTFE to insert the electrodes. The device should be higher than the extracellular solution so that the top end, where the electrodes are exposed, will never come in contact with the solution. For insulation, use epoxy glue on any part of the electrode leads that might be exposed. - Use a square pulse shape with a 50% duty cycle, with no DC component to avoid electrolysis. Vary pulse duration between 10 µs and 4 ms to cause effective stimulation without burning the culture. Ensure that the amplitude is in the order of ± 22 V (see Figure 3). The square pulse can be observed on an oscilloscope connected in parallel to the electrodes.
NOTE: For easy programming of any desired waveform, use a commercial waveform editing software (see Materials list). Enter graphically the desired waveform and send it to the waveform generator.
- Use electrodes made of platinum with a thickness on the order of 0.005'' (127 µm). When used with the 13 mm coverslips, ensure that the distance between the two electrodes is around 11 mm, and position the electrodes 1 mm above the culture.
- To test for field homogeneity use a probe electrode. Use a grid of at least 1 mm x 1 mm to allow the probe to be moved in the area between electrodes and measure electric potential.
- Measure electric potential. Use
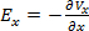 to calculate the electric field. Use one of the electrodes as a reference electrode. Measure the electric field with varying pulse durations between 100 µs to 4 ms (see Figure 3B for an example of 100 µs pulse duration) to verify that the field is homogeneous within the range of stimulating durations.
to calculate the electric field. Use one of the electrodes as a reference electrode. Measure the electric field with varying pulse durations between 100 µs to 4 ms (see Figure 3B for an example of 100 µs pulse duration) to verify that the field is homogeneous within the range of stimulating durations.
NOTE: See Figure 3D for an example of a measured electric field homogeneity when the pulse duration was 1 ms.
- Measure electric potential. Use
Representative Results
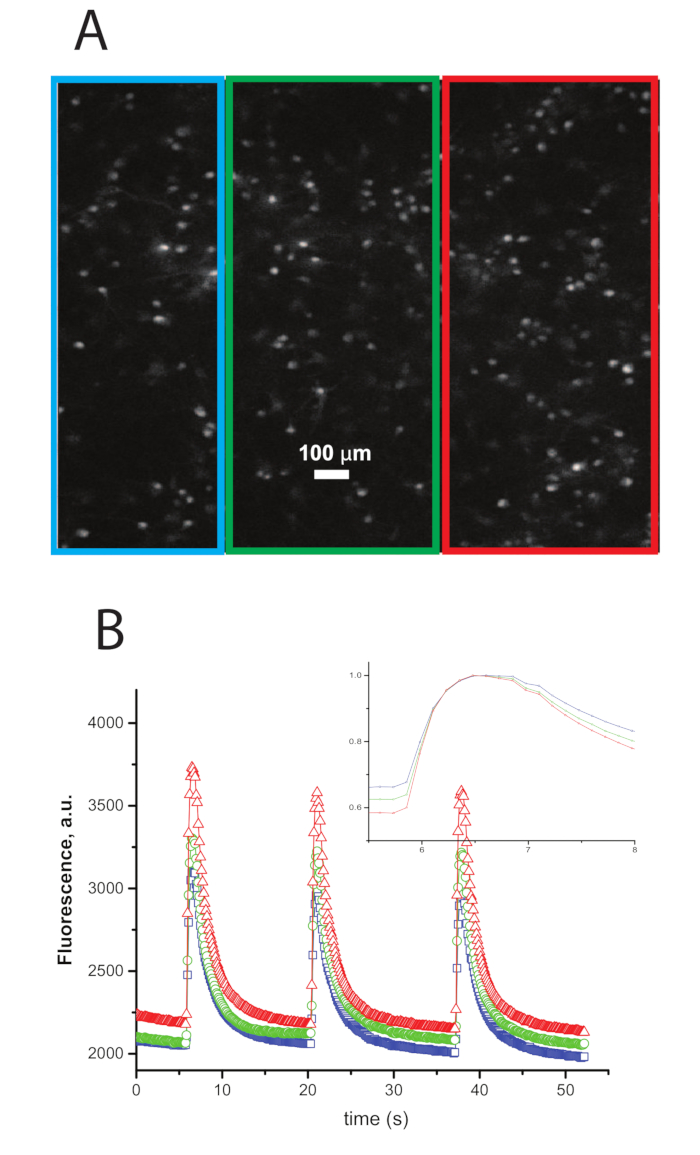
Figure 1: Example Traces of Calcium Transients Imaged During Synchronous Network Bursts. A. An image of neurons that were dyed previous to the experiment with a calcium dye. B. Traces of intensity vs. time of the ROIs in A with the color of the trace representing the color of the border of the ROI in A. A large increase in intensity synchronized within the three ROIs represents a network burst[Insert Figure 2]
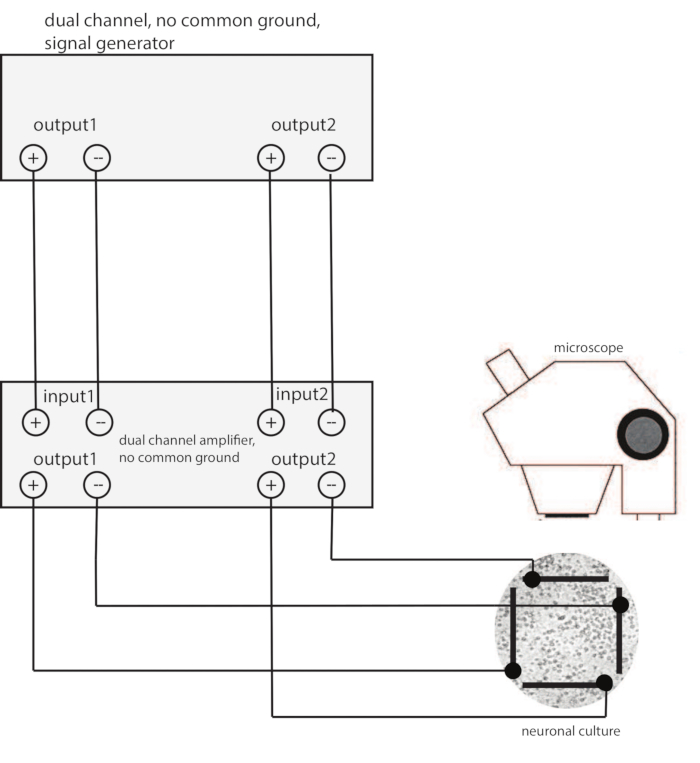
Figure 2: Schematic of the Setup Used for Magnetic Stimulation of Neuronal Cultures. A. At the top is shown the magnetic coil (blue circles), which is located 5 mm concentrically above the neuronal ring culture, placed in a Petri dish (blue outline). A pickup coil (red circle) positioned on the circumference of the Petri dish measures the voltage induced by the magnetic pulse. At the bottom, the measured dynamics of the magnetic stimulator coil are shown (using an MS capacitor voltage load of 5,000 kV), as integrated from the pickup coil. The induced electric field (calculated for a ring radius of 14 mm) is depicted in green while the magnetic field is depicted in blue B. An inverted microscope images fluorescent dyes sensitive to calcium transients of neurons reacting to magnetic pulses. C. Neurons grown on a pattern of concentric rings, used for effective stimulation by the ring magnetic stimulator. D. Bright field microscope image of neurons grown on one line of the pattern
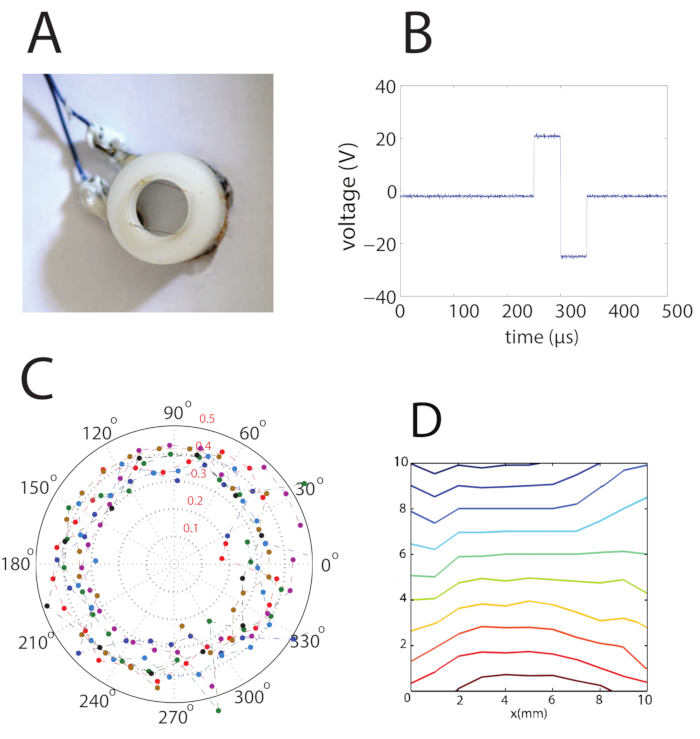
Figure 3: Basic Setup for Electric Stimulation, using One Pair of Parallel Bath Electrodes to Determine that 2D Cultures Have no Preferred Orientation of Electric Stimulation. A. Apparatus used for culture stimulation. The electric field is produced by applying voltage to the platinum wire electrodes. The distance between the two wires is 13 mm. B. An example of a voltage signal to be applied on the electrodes in A. The bipolar shape of the pulse helps prevent electrolysis of the recording solution at the electrodes. C. When stimulating a 2D culture in different rotations of the electrodes, there is isotropy of the neuronal response. D. An example of the electric field measurement using the probe is described in step 2.2. The electric field is uniform to an error of up to 10%.
Offenlegungen
The authors have nothing to disclose.
Materials
| Calcium chloride , 1M | Fluka | 21098 | Extracellular recording solution. |
| D-(+)-Glucose, 1M | Sigma-Aldrich | 65146 | Plating medium, Extracellular recording solution. |
| Hepes, 1M | Sigma-Aldrich | H0887 | Extracellular recording solution. |
| KCl, 3M | Merck | 1049361000 | Extracellular recording solution. |
| Magnesium chloride , 1M | Sigma-Aldrich | M1028 | Extracellular recording solution. |
| NaCl, 4M | Bio-Lab | 19030591 | Extracellular recording solution . |
| Sucrose, 1M | Sigma-Aldrich | S1888 | Extracellular recording solution. |
| Electrodes wires | A-M Systems, Carlsborg WA | 767000 | Electric stimulation of neuronal cultures. |
| Signal generator | BKPrecision | 4079 | Shaping of the electric signal. |
| Amplifier | Homemade | Voltage amplification of the signal from the signal generator to the electrodes. | |
| Power supply | Matrix | MPS-3005 LK-3 | Power supply to the sputtering machine. |
| Platinum wires 0.005'' thick; A-M Systems, | Carlsborg WA | 767000 | Electric stimulation of neuronal cultures. |

