Electrophysiological Recordings For Assessing Neuronal Regenerations in Co-cultured Spinal Cord Slices
Abstract
Source: Heidemann, M., et al. Investigating Functional Regeneration in Organotypic Spinal Cord Co-cultures Grown on Multi-electrode Arrays. J. Vis. Exp. (2015).
This video presents electrophysiological recordings from organotypic co-cultures of spinal cord slices, captured using a multi-electrode array or MEA device. These recordings assess neuronal regeneration between the slices. The synchronized electrical activity observed confirms successful neural regeneration.
Protocol
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review committee.
1. Mounting Spinal Cord Tissue Slices on MEAs
- Warm up sterile nutrient medium in the incubator (37 °C, lightly unscrewed lid for oxygenation).
- Position the multi-electrode array or MEA with sterile tweezers with rubber-covered tips at room temperature (RT) in the petri dish under a stereo microscope with the electrode array in focus. Center a 6 µl droplet of chicken plasma on the clean, dust-free, and sterile electrode array. Using a small spatula, carefully slide two spinal cord sections with ventral sides facing each other into the plasma droplet. Do not touch the electrodes with the spatula.
- Add 8 µl of thrombin around the chicken plasma droplet. Using the thrombin pipette tip, carefully mix and spread the chicken plasma/thrombin mixture around the two slices. Again, do not touch the brittle electrode array directly. Just before coagulation, aspirate excess chicken plasma/thrombin.
- Cap the petri dish to retain high humidity while the MEA/culture assembly sits for about 1 hr in a humidified chamber inside the incubator at 37 °C.
- Carefully add 10 µl of nutrient medium to the culture chamber, cap the petri dish, and put back into the incubator for about 30 min.
- Place each MEA/culture assembly with sterile, rubber-covered tips into a roller tube, add 3 ml of nutrient medium, and tightly close the lid. Place the roller tube in the roller drum, rotating at 1 – 2 rpm in the incubator in a 5% CO2-containing atmosphere at 37 °C.
- Change half of the nutrient medium after 7 days in vitro (DIV) and afterward 1 – 2 times per week.
2. Mechanical Lesions
- Take the MEA/culture assembly with sterile, rubber-covered tips out of the roller tube and place in a petri dish without nutrient medium under a stereo microscope with the tissue in focus. Visually verify that the two slices are fused.
- Hold the MEA/culture assembly steady by placing tweezers with rubber-covered tips on the MEA.
- Place a scalpel blade in the groove of the MEA close to the tissue slices. Hold the scalpel rather horizontally.
- Lift the scalpel handle up but let the scalpel blade stay in the groove of the MEA in such a way that the blade "rolls" from base to tip and thereby cuts through the tissue covering the groove.
- Severe any residual tissue connections with a 25 G needle tip if necessary. Work only in the area within the groove and do not touch the tender edges.
- Put the MEA/culture assembly back into the roller tube. Provide 3 ml of fresh nutrient medium to the cultures and place them back into the roller drum in the incubator.
3. Electrophysiological Recordings of Spontaneous Activity
- To investigate functional regeneration among the two spinal cord slices after the chosen number of DIV, mount the MEA/culture assembly in a recording chamber on a microscope and apply about 500 µl extracellular solution (see materials list).
- Wait 10 min before the first recording to allow the system to stabilize.
- Record basic spontaneous activity 2x for about 10 min from each activity-detecting electrode of the MEA at RT.
- To ensure stable, extracellular conditions, exchange extracellular solution after every recording session.
- To disinhibit the network, apply an extracellular solution containing strychnine (1 μM) and gabazine (10 μM). Wait for at least 2 min before starting the recording.
4. Data Analysis
NOTE: For the detection of the extracellularly recorded action potentials, use a detector based on standard deviations and a subsequent discriminator for each electrode.
- Display the detected neuronal activity of each electrode in a raster plot according to standard procedures (Figure 1F).
- Determine and display the total network activity for each slice by summarizing the number of events in the appropriate channels within a sliding window of 10 msec, shifted by 1 msec steps (Figure 1G).
- Detect in each slice individual bursts (clusters of activity that appear on several electrodes). Therefore, set a minimal peak activity threshold in the corresponding total network activity and define each burst start and burst end. For example, an appropriate threshold is 25% of the average burst amplitude, and a burst start can be defined as being the first event in a time window of at least 5 msec, and a burst end by being the last event in a time window of at least 25 msec.
- To quantify functional regeneration, calculate the percentage of synchronized bursts between the two slices. To do this, calculate the latency between a burst in one slice and the following burst in the other slice.
NOTE: A burst pair is termed "synchronized" when the latency is smaller than the average burst length. Especially in co-cultures with a lot of activity, the possibility exists that both sides coincidentally initiate a burst in the chosen latency window. This fact has to be taken into account in the quantification of the percentage of synchronized bursts.
Representative Results
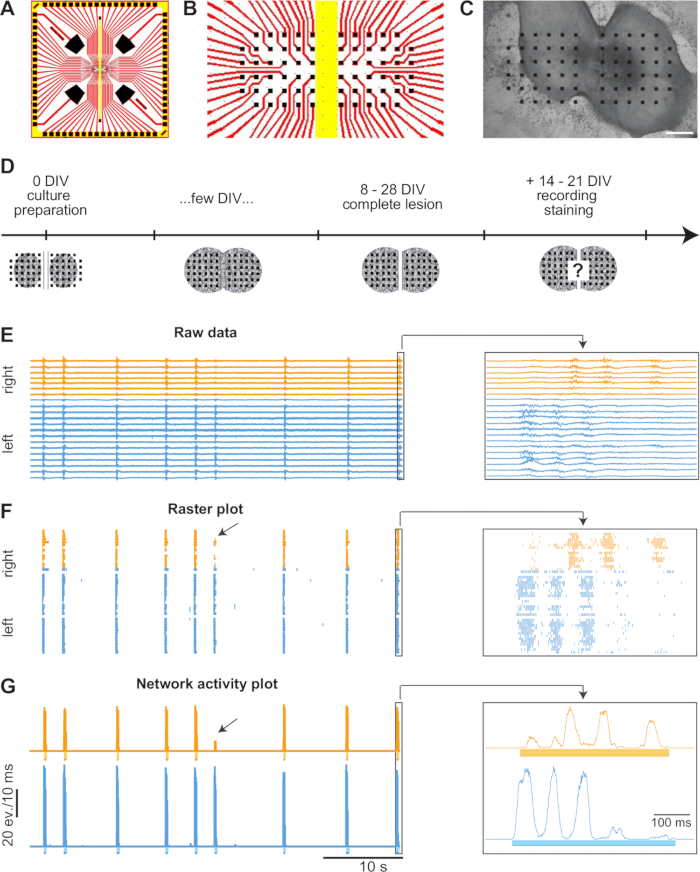
Figure 1. Display and Analysis of Spontaneous Activity. (A) Diagram of a MEA. The platinum-covered electrodes are depicted in black, the transparent wires in red, and the groove in the middle of the MEA in yellow. (B) Close-up of the electrode array located in the center of the MEA. (C) Bright-field image of an 8 DIV old culture. The slices have grown and fused along the sides facing each other. The yellow bar represents the electrode- and insulation-free groove of the MEA. Scale bar = 400 µm (D) Timeline of experiments. Two spinal cord slices of E14 rat embryos are placed next to each other on MEAs. Within a few days, the slices grow and fuse along the sides facing each other. In a time frame of 8 – 28 DIV, complete lesions are performed through the fusion site. Two to three weeks later, the spontaneous activity is recorded, and the cultures are fixed for immunohistochemical stainings. (E) Spontaneous activity traces of each individual electrode of a 23 DIV old culture. For clearer visualization, only every second trace is illustrated. Orange traces depict activity that has been recorded from the slice on the right side and blue traces from the left side. Most of the bursts are synchronized between the two. The arrow points to a burst occurring in the left slice that only partially propagated to the right slice. The activity in the right slice however did not reach the chosen threshold of at least 25% of the averaged maximal peak activity of the according side and therefore, is not detected as a burst. Magnifications on the right depict the last synchronized burst pair. (F) A raster plot of the activity is shown in (E). (G) Network activity plot with defined bursts (bars below baseline) of the activity shown in (E).
Offenlegungen
The authors have nothing to disclose.
Materials
| Planar multielectrode array | Qwane Biosciences | custom-made according to the design of our lab | |
| Nutrient medium | For 100 ml | ||
| Dulbeccos modified Eagle's medium | Gibco | 31966-021 | 80 ml |
| Horse serum | Gibco | 26050-070 | 10 ml |
| distilled, sterile water | 10 ml | ||
| Nerve growth factor-7S [5ng/mL] | Sigma-Aldrich | N0513 | 200 µl |
| reconstituted in wash solution with 1% BSA | |||
| Extracellular matrix gel | BD Biosciences | 356230 | Must stay cold at all times; dilute 1:50 with medium optimized for prenatal and embryonic neurons |
| Extracellular solution (pH 7.4) | [mM] | ||
| NaCl | 145 | ||
| KCl | 4 | ||
| MgCl2 | 1 | ||
| CaCl2 | 2 | ||
| HEPES | 5 | ||
| Na-pyruvate | 2 | ||
| Glucose | 5 | ||
| Gabazine | Sigma-Aldrich | SR-95531 | |
| Micropipette | World Precision Instruments, Inc. | MF28G-5 | |
| Strychnine | Sigma-Aldrich | S0532 | toxic |
| Tissue culture flat tube 10 (= roller tube) | Techno Plastic Products AG | 91243 |

