Neuronal Enrichment of Dorsal Root Ganglia by Immunopanning
Abstract
Source: Maguire, A. D., et al. Enrichment of Adult Mouse Dorsal Root Ganglia Neuron Cultures by Immunopanning. J. Vis. Exp. (2023)
This video demonstrates a protocol for enriching neuronal cultures from dorsal root ganglia by immunopanning, thereby improving their purity and utility for neurological research.
Protocol
1. Experimental method
- Day 1: preparation of dishes
- In a biosafety cabinet, coat the desired culture surface with enough poly-D-lysine to cover the bottom of the well. Store overnight at 4 °C. Here, 24-well glass-bottom plates were used for imaging, and thus 300 µL of poly-D-lysine was used.
- In a biosafety cabinet, prepare the panning dishes: for the CD45 dish, add 10 mL of working tris-HCl solution and 30 µL of goat anti-rat IgG (immunoglobulin G) to a 10 cm Petri dish; for the PDGFRβ (platelet-derived growth factor receptor beta) dish, add 10 mL of working tris-HCl solution and 30 µL of goat anti-rabbit IgG to a 10 cm Petri dish; for the O4 hybridoma dish, add 10 mL of working tris-HCl solution and 30 µL of goat anti-mouse IgG to a 10 cm Petri dish. Ensure the solution covers the entire dish area and leave it at 4 °C overnight.
- Day 2: dissection, trituration, and immunopanning
- Aspirate the poly-D-lysine, wash the plates three times with tissue culture water, tilt the lid open, and allow the surface to dry completely in a biosafety cabinet. Apply enough laminin to the plates to coat the bottom of each well, and place in an incubator at 37 °C. For the 24-well plates, 200 µL is sufficient.
- Finish preparing the panning dishes. Wash each dish with D-PBS and incubate with 10 µg of primary antibody. Allow to sit at room temperature for at least 2 h.
- For the CD45 dish, add 5 mL of 0.2% BSA (bovine serum albumin) and 20 µL of CD45 primary; for the PDGFRβ dish, add 5 mL of 0.2% BSA and 15.4 µL of PDGFRβ; for the O4 dish, add 3 mL of 0.2% BSA, 2 mL of O4 hybridoma, and an additional 100 µL of 4% BSA (to ensure the final BSA concentration remains at 0.2%).
- Euthanize one to four mice using 0.1 mL/kg sodium pentobarbitol (per 10 cm immunopanning dish). We used C57BL/6 mice at 8-10 weeks old. Both sexes were used and cultured separately. Perfuse the animal with 30 mL of cold 0.9% saline.
- Pin the front and hind paws to a styrofoam stage and use a clean razor blade to expose the spinal column from the back. Perform a laminectomy by making cuts about halfway deep on either side of the dorsal spinal column, removing the dorsal half of the column to expose the spinal cord. Either gently cut the nerves and remove the spinal cord or gently push the cord to the side, maintaining the nerve integrity.
- Use the spinal nerve roots (either severed or attached to the spinal cord) to find and remove all the DRGs (dorsal root ganglions) from either side of the spinal column. Trim the nerve debris from each DRG as it is removed (more nerve debris in the culture may lead to poor culture survival). Collect the DRGs in 15 mL of HBSS (Hank’s balanced salt solution in a 15 mL conical tube on ice.
NOTE: Note that the tissue is dissected in open air, but once the DRGs are added to the tube, they should be treated as sterile and only manipulated in a biosafety cabinet. - Place frozen stocks of stemxyme 1 and 0.4% DNAse in a water bath approximately 30 min prior to the end of the dissections (roughly when starting the last mouse). Allow them to settle to the bottom of the conical tube, then continue the protocol in a biosafety cabinet.
- Aspirate most of the HBSS-/- from the DRGs. Use a pipette rather than suction to get <1 mL of liquid, and leave ~100 µL liquid so as not to risk losing tissue.
- Wash three times with 1 mL of HBSS-/-. Add 5 mL of working stemxyme solution to the tissue. Cover the lid with a transparent film and float the tube on its side in a water bath set to 37 °C for 1 h.
- After incubation in the enzyme, add 1 mL of low ovo inhibitor solution to the tube. Triturate the cells gently with a p1000 pipette 10-15 times. Allow the tissue chunks to settle.
- Transfer the top 2-3 mL of solution (containing dissociated cells) to fresh low ovomucoid solution. Repeat until the tissue is fully dissociated and no visible chunks remain.
- Centrifuge for 10 min at 300 x g at room temperature. Siphon off the supernatant again, using a pipette rather than suction for the last 1 mL, leaving ~100 µL, and resuspend the cell pellet in 1 mL of panning buffer.
- Gently pipette to mix. Pre-wet a 70 µm cell strainer with 1 mL of panning buffer over a 50 mL conical tube. Filter the cell solution through the cell strainer.
- Wash the tube with 1 mL of panning buffer, then pass through the strainer (3 mL of filtrate). Layer the cell suspension gently (1 mL at a time) on top of 2 mL of a 15% BSA cushion to remove myelin debris. A 2 mL cushion is effective for two mice, and 3 mL is effective for four mice.
- For layering, place the 2 mL of BSA in the tube, close it, and gently coat the sides of the tube with BSA. Then, gently layer the cell suspension on top by pipetting it against the side of the tube.
- Centrifuge at room temperature at 300 x g for 10 min (slow acceleration and deceleration). Use a P1000 pipette to remove the clear liquid on top, the myelin phase in between, and the BSA, 1 mL at a time (changing tips in between each mL). Leave ~100 µL of BSA so as not to disturb the pellet.
- Add 5 mL of panning buffer and incubate the tube in a 37 °C, 10% CO2 incubator for 30-45 min. This allows for antigen retrieval. Be sure to loosen the cap to allow gas exchange.
- Wash the CD45 dish three times with D-PBS. Pour off the final rinse. Decant the cell suspension into the CD45 dish.
- Incubate the cells on the CD45 dish for a total of 20 min at room temperature, swirling gently at the 10 min point to allow the cells equal access to the antibody.
- Rinse the PDGFRβ dish three times with D-PBS and pour off the final rinse. Transfer the unbound cells from the CD45 dish to the PDGFRβ dish.
- Gently shake the CD45 dish and prop it up at an angle. Gently pipette 1 mL of the cell suspension over the dish to collect unbound cells. Decant it into the PDGFRβ dish and pipette to transfer the remaining cell suspension to the PDGFRβ plate.
- Incubate the PDGFRβ plate for a total of 20 min at room temperature, swirling gently at the 10 min point to allow the cells equal access to the antibody.
- Rinse the O4 dish three times with D-PBS and pour off the final rinse. Transfer the unbound cells from the PDGFRβ dish to the O4 dish using the same method as in step 1.2.19. Incubate the O4 plate for a total of 20 min at room temperature, swirling gently at the 10 min point to allow the cells equal access to the antibody.
- Transfer the cell suspension to a 15 mL conical tube and centrifuge for 10 min at 300 x g. Resuspend the cells in the desired volume and dilute 1:1 with trypan blue for cell viability. Count medium-to-large cells with a hemocytometer (this will allow for the best representation of the number of neurons in the culture).
- Remove the laminin and plate the cells at the desired density. Do not allow the plate to dry between laminin removal and plating, and add the cells immediately.
- Culture the quantified cells for neuronal enrichment (Figure 1) with 500 neurons in 25 µL of neural medium (as described below) per well in 24-well plates and incubate at 37 °C for 30 min. Flood the wells to a final volume of 500 µL.
- Prepare 50 mL of neuron media by adding 23.2 mL of DMEM, 23.2 mL of neurobasal medium, 500 µL of glutamax, 500 µL of sodium pyruvate, 500 µL of penicillin/streptomycin (aliquot immediately upon arrival and store at -20 °C), 500 µL of insulin (5 µg/mL final), 500 µL of SATO, 50 µL of N-acetyl-L-cysteine (NAC; 5 µg/mL final), and 1,000 µL of B27+ (immediately aliquot and store at -20 °C upon receiving).
- Prepare the insulin stock by dissolving it in culture-grade water to a concentration of 0.5 mg/mL (50 mg in 100 mL), adjusting the pH to 7.4, filter sterilizing (0.22 µm), preparing 500 µL aliquots, and storing at -20 °C.
- Prepare the SATO stock by combining 20 µL of progesterone (2.5 mg in 100 µL of ethanol), 800 µL of sodium selenite (4 mg in 10 mL of neurobasal media + 10 µL of 1 N NaOH (Sodium hydroxide)), 800 mg of BSA, 800 mg of transferrin, 128 mg of putrescine dihydrochloride, and 80 mL of neurobasal. Filter sterilize (0.22 µm), prepare 500 µL aliquots, and store at -20 °C. It is essential to make fresh progesterone and sodium selenite solutions.
- Prepare 50 µL of N-acetyl-L-cysteine (NAC) stock by dissolving in neurobasal medium to a stock concentration of 5 mg/mL (50 mg in 10 mL), filter sterilize (0.22 µm), aliquot, and store at -20 °C.
- Prepare 50 mL of neuron media by adding 23.2 mL of DMEM, 23.2 mL of neurobasal medium, 500 µL of glutamax, 500 µL of sodium pyruvate, 500 µL of penicillin/streptomycin (aliquot immediately upon arrival and store at -20 °C), 500 µL of insulin (5 µg/mL final), 500 µL of SATO, 50 µL of N-acetyl-L-cysteine (NAC; 5 µg/mL final), and 1,000 µL of B27+ (immediately aliquot and store at -20 °C upon receiving).
Representative Results
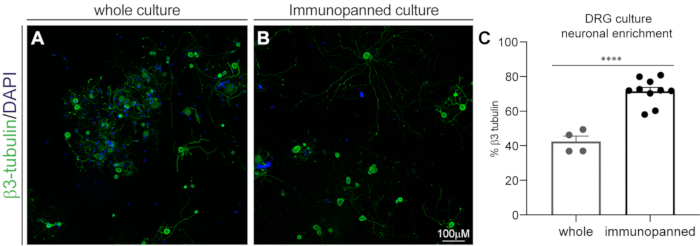
Figure 1: DRG culture neuronal enrichment by immunopanning. Fixed cultures were stained with β3-tubulin for neurons and DAPI for all nuclei. (A) Representative image of a whole (non-immunopanned) culture. (B) Representative image of an immunopanned culture. (C) Quantification of β3-tubulin-positive cells as a percentage of total DAPI-positive cells. Bars indicate mean ± standard error mean (SEM). **** = p < 0.0001, unpaired t-test.
Offenlegungen
The authors have nothing to disclose.
Materials
| 0.2 um Syringe filters | Fisher | 723-2520 | |
| 100 mm petri dishes | ThermoFisher | FB0875712 | |
| 24 well black glass-bottom plates | Cellvis | P24-1.5H-N | |
| 70 um cell strainer | Cedarlane | 15-1070-1(BI) | |
| B27+ supplement | Gibco | A3582801 | |
| Bovine Serum Albumin | Sigma Aldrich | A7906 | for 15% BSA cushion, and ICC (heat shock fraction ≥98%) |
| Bovine Serum Albumin | Sigma Aldrich | A4161 | for 4% BSA for immunopanning, and SATO for media (essentially globulin free, suitable for cell culture, ≥99%) |
| CD45 antibody | BD Pharmigen | 550539 | |
| DAPI | Invitrogen | D1306 | |
| DMEM | Gibco | 11960069 | |
| DNAse | Worthington | LS002007 | for stemxyme solution and panning buffer |
| D-PBS | Sigma Aldrich | D8662 | |
| EBSS | Sigma Aldrich | E6267 | for DNAse solution |
| Filter paper P8 grade | ThermoFisher | 09-795K | for 8% PFA |
| Glutamax | ThermoFisher | 35050061 | |
| goat-anti-mouse IgG | Jackson Immunoresearch | 115-005-020 | for O4 dish |
| goat-anti-rabbit 488 | Invitrogen | A11008 | |
| goat-anti-rabbit IgG | Jackson Immunoresearch | 115-005-003 | for PDGFRB dish |
| goat-anti-rat IgG | Jackson Immunoresearch | 115-005-044 | for CD45 dish |
| HBSS -/- | Sigma Aldrich | 14175145 | |
| Insulin | Sigma Aldrich | I2643 | |
| laminin | Invitrogen | 23017-015 | |
| N-acetyl cysteine | Sigma Aldrich | A8199 | |
| Neurobasal | Gibco | 21103049 | |
| Normal Donkey Serum | Sigma-Aldrich | D9663 | |
| O4 antibody | n/a | n/a | Hybridoma |
| Ovomucoid trypsin inhibitor | Cedarlane | LS003086 | for low ovo |
| paraformaldehyde prills | Sigma Aldrich | 441244 | for 8% PFA |
| PDGFRB antibody | Abcam | AB32570 | |
| penicillin/ streptomycin | Gibco | 15140-122 | |
| Poly-D-Lysine | Sigma Aldrich | P6407 | |
| Progesterone | Sigma Aldrich | P8783 | for SATO |
| Putrescine dihydrochrloride | Sigma Aldrich | P5780 | for SATO |
| Sodium phosphate dibasic | Fisher | S374-1 | for 0.2 M PB |
| Sodium Phosphate monobasic dihydrate | Sigma Aldrich | 4269 | for 0.2 M PB |
| Sodium Pyruvate | ThermoFisher | 11360070 | |
| Sodium Selenite | Sigma Aldrich | S5261 | for SATO |
| Stemxyme I | Cedarlane | LS004107 | for tissue dissociation; combination collagenase |
| Transferrin | Sigma Aldrich | T1147 | for SATO |
| Tris-HCl | Millipore Sigma | T5941 | |
| trypan blue | Gibco | 15250-061 | |
| β3-Tubulin | Sigma-Aldrich | T2200 |

