Isolating and Culturing Cells from Diffuse Intrinsic Pontine Glioma
Abstract
Source: Lin, G. L. et al., A Protocol for Rapid Post-mortem Cell Culture of Diffuse Intrinsic Pontine Glioma (DIPG). J. Vis. Exp. (2017)
This video demonstrates the process of isolating and culturing glial progenitor cells from diffuse intrinsic pontine glioma, or DIPG-affected brainstem tissue. Glial cells isolated from tumor tissue via an enzymatic digestion are cultured in a neurobasal medium, which promotes their growth and the development of free-floating neurospheres.
Protocol
All procedures involving human participants have been performed in compliance with the institutional, national, and international guidelines for human welfare and have been reviewed by the local institutional review board.
1. Obtaining Tissue Samples
NOTE: A critical component of this protocol requires proper handling of the tissue donation starting immediately at the time of autopsy. The overall sample viability depends significantly on minimizing the Post-Mortem Interval (PMI), immediately transferring the tissue into the appropriate cold medium supplemented with antibiotics/antimycotics, keeping the sample on wet ice throughout transportation, and maintaining sterile technique throughout the protocol.
- Upon notification of an imminent donation, prepare a sample collection kit. Using a shipping box with an insulated container that can be directly used for return transportation of the tissue sample, include the following materials:
- Include materials for sterile preparation, including sterile gloves, drapes, and scalpels.
- Include 8 – 10 sterile 50 mL conical tubes containing 30 mL shipping media in the insulated container with ice or cold packs.
NOTE: While any standard cell culture media can work, the best sample viability is obtained with Hibernate-A supplemented with antibiotic/antimycotic. - Include any further sample tubes for other analyses (e.g., fixatives).
- If the autopsy will not be performed at the investigator's site, include a document that clearly states how to collect the tumor sample. This includes details such as maintaining sterility, keeping sample tubes on wet ice, and including samples from the midbrain and medulla as tumor cells often invade these regions.
- Maintain sterility during the autopsy. Consider the brain sterile inside the cranium, so observe sterile technique (including changing gloves) once the cranium is open. Avoiding contamination with skin and hair is critical.
- Dissect the tumor from the ventral side (the "belly" of the pons, see Figure 1). With a sterile scalpel, cut small 1 cm chunks from the tumor and immediately transfer into cold shipping media (see NOTE in step 1.1.2) and put on wet ice. Place 10 mL of tissue into each tube, resulting in a final volume of tissue and media in each tube of 40 mL.
NOTE: In the case of diffuse intrinsic pontine glioma (DIPG), the tumor diffusely infiltrates the pons and frequently the rest of the brainstem. As such, collect as much of the sample as possible, typically including 3 – 4 tubes (30 – 40 mL tissue) from pons and 1 – 2 tubes (10 – 20 mL tissue) each from midbrain and medulla. - Collect samples from the midbrain and medulla juxtaposed to the pons, which often contain many tumor cells.
- After sample collection, ship the samples overnight (O/N) on wet ice in the insulated container. Do not ship the sample on dry ice, as freezing will kill the cells.
2. Preparation for Sample Processing
- Prior to beginning sample preparation, sterilize tissue culture hoods along with razor blades, curved hemostats, and any other non-sterile tools under ultraviolet (UV) light for 1 h. Perform all of the following steps under sterile conditions to prevent contamination of the cultures.
- Prepare and sterile filter solutions.
- To prepare 1.8 M sucrose solution, dissolve 308.07 g sucrose in 300 mL distilled water. Add 50 mL 10x Hank's-buffered Saline Solution (HBSS) without calcium or magnesium and bring total volume to 500 mL using distilled water. Store at 4 °C.
- To prepare enzymatic digestion solution, take 50 mL HBSS with calcium and magnesium for every 10 mL of minced tissue and add 500 μL 5 mg/mL deoxyribonuclease I (final concentration 50 μg/mL), 500 μL 2.5 mg/mL collagenase I/II and dispase solution (final concentration 25 μg/mL), and 500 μL 1 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer. Prewarm digestion solution in a 37 °C water bath.
- To prepare tumor stem media (TSM) base, mix 250 mL Neurobasal-A, 250 mL Dulbecco's Modified Eagle Medium: Nutrient Mixture F12 (DMEM/F12), 5 mL 100x antibiotic-antimycotic, 5 mL 200 mM L-alanyl-L-glutamine dipeptide (GlutaMAX-A), 5 mL HEPES buffer, 5 mL 100 mM sodium pyruvate, and 5 mL 100x MEM non-essential amino acids.
- To prepare complete TSM with growth factors, add the following supplements to TSM base: 50x B27 Supplement Minus Vitamin A (1:50), human Epidermal Growth Factor (H-EGF, 20 ng/mL), human Fibroblast Growth Factor (H-FGF, 20 ng/mL), human Platelet-derived Growth Factor AA (H-PDGF-AA, 10 ng/mL), human Platelet-derived Growth Factor BB (H-PDGF-BB, 10 ng/mL), and heparin solution (2 μg/mL).
- Precool a laboratory centrifuge equipped with a swinging-bucket rotor to 4 °C.
3. Mechanical Dissociation
- Transfer the tissue into a high-walled 100 mm x 20 mm cell culture dish. Remove media leftover from shipping and replace with 10 – 15 mL cold culture media.
- Using the curved hemostats to grasp a razor blade, mince the tissue finely while removing obvious blood vessels or meninges.
NOTE: The final tissue fragments should be smaller than 1 mm (see Figure 2B). - Transfer the tissue into a clean 50 mL conical tube. Wash the cell culture dish with an additional 5 mL cold culture media and transfer to the conical tube. Repeat this wash step as necessary to transfer remaining tissue.
- Using a 10 mL serological pipet, triturate gently (4 – 5 times). Allow larger tissue fragments to settle to the bottom of the tube. If necessary, briefly centrifuge the sample for 1 min (350 x g, 4 °C).
- Collect the mechanically dissociated fraction.
- Remove the supernatant and filter it through a 100 μm filter into a new 50 mL conical tube labeled "Mechanical Dissociation." Invert the filter over the original conical tube and wash with culture media to recover tissue fragments.
- Centrifuge the "Mechanical Dissociation" fraction for 5 min (350 x g, 4 °C).
- Remove the supernatant from the pelleted "Mechanical Dissociation" fraction and resuspend the tissue in cold culture media. If there is more than 5 mL of tissue in the "Mechanical Dissociation" conical tube, split the tissue into other 50 mL conical tubes such that no tube has more than 5 mL tissue.
- Store the mechanically dissociated fraction on ice until the sucrose gradient centrifugation step (Step 5). Alternatively, the mechanically dissociated fraction can proceed to Step 5 during the enzymatic dissociation incubation period (Step 4.4).
4. Enzymatic Dissociation
- If there is more than 5 mL of tissue in the tube containing the remaining larger tissue fragments, split the tissue into other new 50 mL conical tubes such that no tube has more than 5 mL tissue.
- Centrifuge the conical tube(s) containing the remaining tissue fragments for 5 min (350 x g, 4 °C).
- Remove the supernatant and add the prewarmed enzymatic digestion solution, such that there is 5 mL digestion solution for every 1 mL tissue (e.g., 25 mL digestion solution for 5 mL tissue).
- Seal the conical tube lids with laboratory film and incubate the reaction on a rotator at 37 °C for 30 min.
- After the incubation, triturate the samples gently (see Figure 2C).
- Using a 10 mL serological pipet, pipet the sample up and down 6 – 8 times. Avoid generating excessive air bubbles.
- Add a 1,000 μL pipet tip to the end of the pipet and triturate an additional 6 – 8 times.
- Allow any remaining chunks to settle to the bottom of the tube. Remove and filter the supernatant with the cells still suspended through a 100 μm filter into a new 50 mL conical tube labeled "Enzymatic Dissociation" and store on ice.
- If significant tissue fragments remain, add an additional 10 mL HBSS with calcium and magnesium to the fragments and repeat steps 3.5.1 and 3.5.2. Filter this final solution through a 100 μm filter into the "Enzymatic Dissociation" tube.
- Centrifuge the "Enzymatic Dissociation" tube for 5 min (350 x g, 4 °C) and continue to sucrose gradient centrifugation.
5. Sucrose Gradient Centrifugation
- If samples are still suspended in solution, centrifuge for 5 min (350 x g, 4 °C).
- Remove the supernatant and resuspend tissue in 20 mL cold HBSS without calcium and magnesium. Bring volume up to 25 mL total with cold HBSS.
- Slowly add 25 mL 1.8 M sucrose solution and invert the tube to mix. This results in a 0.9 M sucrose gradient.
- Centrifuge with no brake for 10 min (800 x g, 4 °C). Using a centrifugation brake will disrupt the gradient and reduce yield (see Figure 2D for an example of a sample before and after centrifugation).
- Carefully aspirate myelin debris and as much sucrose solution as possible. Wash the sample by adding 30 mL cold HBSS without calcium and magnesium and mixing gently. Centrifuge for 5 min (350 x g, 4 °C).
6. ACK Red Blood Cell Lysis
- Remove the wash supernatant. Add 5 mL ACK lysis buffer and gently resuspend the cell pellet, swirling the tube for 1 min at RT.
- Quench the lysis by adding 30 mL cold HBSS without calcium and magnesium.
- Centrifuge for 5 min (350 x g, 4 °C).
7. Initial Culture Maintenance
- Resuspend the final cell pellets in 10 – 15 mL warm complete TSM with growth factors and quantify viable cell density on a hemocytometser using trypan blue exclusion.
NOTE: The range of viable cells varies widely depending on the circumstances of the tissue donation, and quantification can be difficult at this early stage due to leftover cellular debris. In an ideal case, aim to have one million live cells per sample. In cases where excessive debris remains, plate at a lower density in a T175 flask. - Transfer the final cell suspensions to a new T75 culture flask. Spike in additional growth factors every other day to maintain overall growth factor levels and monitor for development of tumor cell neurospheres (see Figure 2E and Figure 3).
Representative Results
Figure 1. Autopsy Sample of a Pontine Tumor. Immediate post-mortem appearance of a diffuse intrinsic pontine glioma. The tumor appears as a large, myelin-rich mass on the ventral surface of the pons.
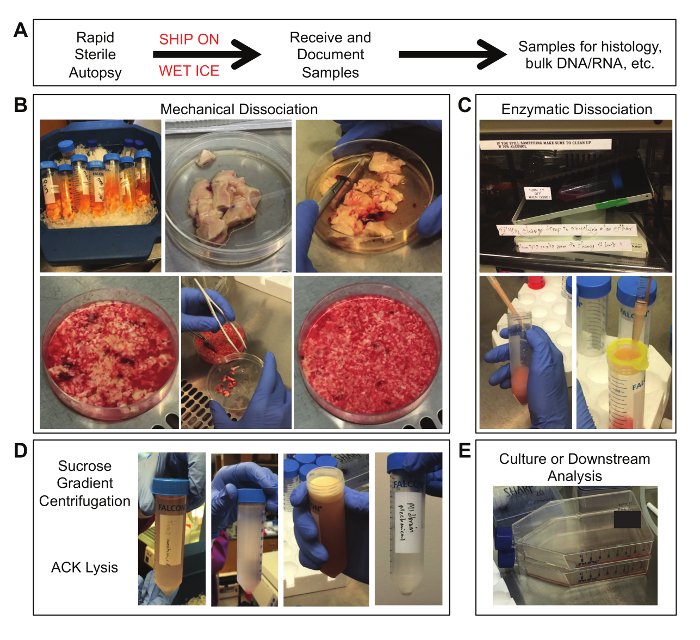
Figure 2. Tissue Samples at Various Stages of Processing. (A) Obtain the sample through sterile autopsy procedures and overnight shipping on wet ice. (B) From left to right: (row 1) tubes containing tumor tissue in shipping media; fresh tissue in a 100 mm x 20 mm cell culture dish; initial mincing of tissue; (row 2) partially minced tissue; removal of meninges and blood vessels; final size of minced tissue pieces. (C) From left to right: (row 1) tissue incubated on a rotating table in a 37 °C oven; (row 2) trituration of tissue through a 1000 μL pipet tip attached to a 10 mL pipet; filtering of dissociated tissue through a 100 μm nylon mesh filter. (D) From left to right: dissociated tissue suspended in 0.9 M sucrose solution prior to centrifugation; dissociated tissue separated across a sucrose gradient after centrifugation; a visible layer of myelin debris on top of the sucrose gradient; a final cell pellet after ACK lysis and wash. (E) The final sample can be placed into culture or used in other downstream analyses.
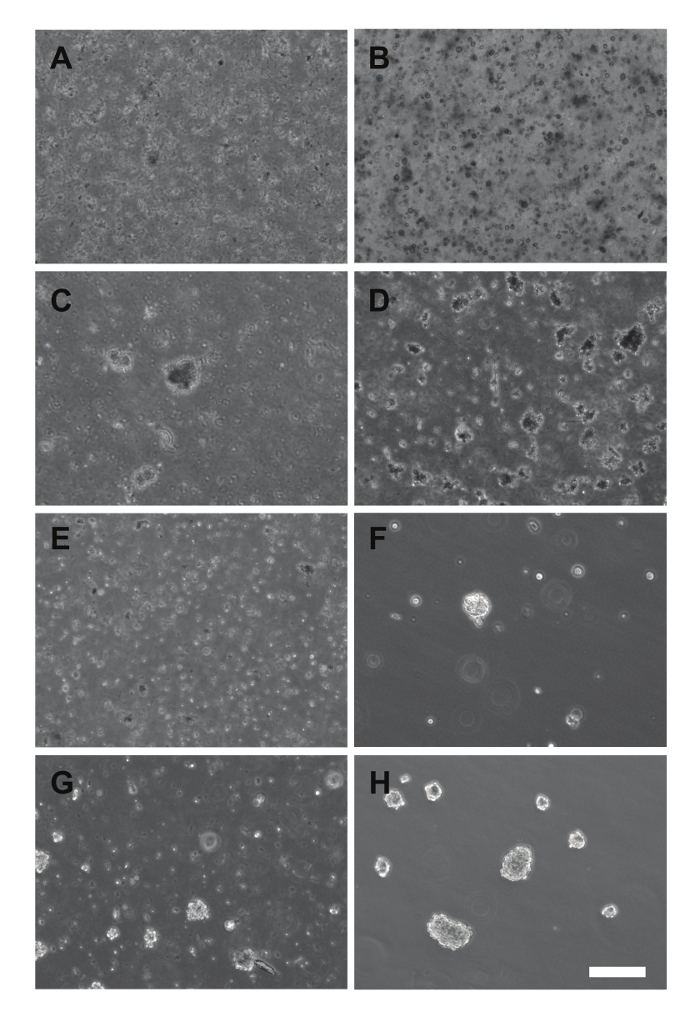
Figure 3. Images of Primary Cultures and Early Passage Cells. (A – B) Cultures plated immediately after dissociation (SU-DIPG-XXX, A), or that have not grown neurospheres yet (SU-DIPG-XXIX, B). (C – D) Early appearance of neurospheres in primary cultures of SU-DIPG-XXVIII (C) and SU-DIPG-XXIX (D). (E-F) First passage of the primary culture from D using a 100 μm nylon filter, with the filtrate (E) and reverse filtrate (F) plated. (G – H) Mature neurospheres from the first passage of SU-DIPG-XXVII (G) and the third passage of SU-DIPG-XXV (H) growing in culture. Scale bar = 200 μm.
Offenlegungen
The authors have nothing to disclose.
Materials
| Hibernate-A | Gibco | A12475-01 | |
| HBSS with Calcium/Magnesium | Gibco | 24020-117 | |
| HBSS | Corning | 21-022-CV | |
| HEPES | Gibco | 15630-080 | |
| Liberase DH (Collagenase/Dispase) | Roche | 5401054001 | |
| DNAse I | Worthington Biochemical | LS002007 | |
| Sucrose | Sigma | S0389 | |
| Antibiotic/Antimycotic | Gibco | 15240-096 | |
| Neurobasal-A | Gibco | 10888-022 | |
| DMEM/F12 | Gibco | 11330-032 | |
| GlutaMAX-I (100X) | Gibco | 35050-061 | |
| Sodium Pyruvate (100mM) | Gibco | 11360-070 | |
| B27 Supplement Minus Vitamin A | Gibco | 12587-010 | |
| MEM Non-Essential Amino Acids (100X) | Gibco | 11140-050 | |
| Human PDGF-AA | Shenandoah Biotechnology | 100-16 | |
| Human PDGF-BB | Shenandoah Biotechnology | 100-18 | |
| Human EGF | Shenandoah Biotechnology | 100-26 | |
| Human FGF | Shenandoah Biotechnology | 100-146 | |
| Sterile scalpel blades | Bard Paker | 372610 | |
| Curved hemostats | Fine Science Tools | 13013-14 | |
| Razor blades | VWR | 55411-050 | |
| 100 x 20 mm cell culture dish | Corning | 353003 | |
| Sterile forceps | TWD Tradewinds | DF8988-5 | |
| Sterile drapes | 3M | 2037 | |
| Sterile gloves | Kimberly Clark | 1182307 | |
| ACK Lysis Buffer | Gibco | A1049201 | |
| 100 micron mesh filter | Fisher | 352360 | |
| 50 mL conical tube | Fisher | 1495949A |

