Differentiating Human Embryonic Stem Cells into Oligodendrocytes
Abstract
Source: Assetta, B., et al. Generation of Human Neurons and Oligodendrocytes from Pluripotent Stem Cells for Modeling Neuron-Oligodendrocyte Interactions. J. Vis. Exp (2020).
This video demonstrates the process of generating oligodendrocyte precursor cells (OPCs) from human embryonic stem cells, followed by the maturation of OPCs to oligodendrocytes. This process of generating oligodendrocytes opens numerous avenues to improve research and treatment of neurodegenerative diseases, including Alzheimer's.
Protocol
1. Human oligodendrocyte precursor cell (OPC) induction from pluripotent stem cells and oligodendrocyte maturation
- Neural Progenitor Cell (NPC) generation: monolayer protocol (~7 days). See Figure 1A for the flow diagram.
- Use commercially available H1 human ES cells at the passage of 52 (see Table of Materials). Culture the cells on extracellular matrix solution-coated 6-well plates (~0.5 mg of matrix solution per 6-well plate; see Table of Materials) using ES cell maintenance medium (see Table of Materials) and incubate the plates at 37 °C with 5% CO2.
- Trans-differentiate the cells into neural progenitor cells (NPCs) by an established approach called dual SMADi, with small molecule inhibitors for multiple signaling pathways. Here we use a widely accepted commercial kit and follow the monolayer protocol provided by the manufacturer (see Table of Materials).
- On Day -1, plate 0.5–1 x 106 cells per well in a 6-well plate coated by a growth factor reduced matrix solution (see Table of Materials; ~0.5 mg of matrix solution per 6-well plate) with embryonic stem (ES) cell maintenance medium (see Table of Materials). This growth factor-reduced matrix solution is used to coat all the plates that will be used in the following steps.
- On Day 0, treat cells for 24 h with ES cell maintenance medium (see Table of Materials) supplemented by 2% dimethyl sulfoxide (DMSO).
- On Day 1–6, change the full media with a warm (37 °C) neural induction medium containing the SMAD inhibitors from the commercial kit (see Table of Materials). If cells divide and reach confluence before Day 7, passage them to the seeding density of 0.5–1 x 106, as described earlier in step 1.1.3.
- On Day 7, passage NPCs using cell detachment solution (see Table of Materials) and plate at a seeding density of 1–2 x 105 cells/well of a 24-well plate.
- Assay differentiation efficiency by immunohistochemical (IHC) staining for the absence of pluripotency markers, such as OCT4, and the presence of NPC markers, such as PAX6, Nestin, and Sox1.
- At this stage, detached NPCs can be frozen in the specialized commercial NPC freezing media (see Table of Materials) and stored in liquid nitrogen for up to 3 months. After freeze-and-thaw for once, NPCs still retain the multipotency to give rise to neurons, astrocytes, and OPCs with reliable protocols.
- Oligodendrocyte precursor cell (OPC) generation (~7 days). Please see Figure 1A for the flow diagram.
- On Day 7, passage NPCs using cell detachment solution (see Table of Materials) and plate them at a seeding density of 1–2 x 105 cells per well in a 24-well plate in warm (37 °C) neural induction medium plus SMAD inhibitors from the commercial kit (see Table of Materials).
- On Day 8, prepare a solution of 1% DMSO in the OPC differentiation medium and treat the plated NPCs for 24 h. The OPC differentiation medium is composed of: basal (DMEM/F12) medium, 1% N2 supplement, 1% B27 supplement, basic fibroblast growth factor (bFGF) at 20 ng/mL, smoothened agonist (SAG) at 1 µM, platelet-derived growth factor (PDGF-AA) at 10 ng/mL (see Table of Materials).
- On Day 9, replace media with fresh OPC differentiation medium without DMSO. Feed the cells every other day until Day 15. If the cells reach confluence before Day 15, passage them to the seeding density of 1–2 x 105 cells per well, as described in step 1.2.1.
- On Day 14, plate OPCs in OPC differentiation medium at a density of 1–2 x 105 cells/well in a 24-well plate.
- At this stage (Day 15), test cells for the presence of OPC-specific markers by immunohistochemistry (IHC) staining or quantitative polymerase chain reaction (qPCR) (e.g., O4, Olig1/2, CSPG4/Ng2, NKX2.2, PDGFRa; Figure 1B) and for the absence of NPC markers (Pax6 or Nestin; Figure 1D). We typically detect the O4 immunoreactivity in more than 95% of the cells on Day 15. Of particular relevance to Alzheimer's disease, the expression of APP (amyloid precursor protein), BACE1 (the processing protease β-secretase 1), and peptide amyloid-β (Aβ) are abundant in OPCs (Figure 1F).
- Oligodendrocyte (OL) maturation (~7–20 days)
- On Day 15, replace media with OL maturation medium: Neurobasal-A medium, 2% B27 supplement, 1 µM cyclic adenosine monophosphate (cAMP), 200 ng/mL T3 triiodothyronine, and Clemastine of 1 µM (see Table of Materials). Change the medium every other day or every day, if necessary.
- When cells reach 90% confluence, split at a 1:3 ratio up to 2 passages or until cell division slows down substantially. If OPCs divide too fast and reach confluency in less than 3 days, add Ara-C (see Table of Materials) at a concentration of 2–5 µM for 1–3 days. Active proliferation indicates lowered maturation efficiency.
- Examine the efficiency of oligodendroglial maturation by assessing the expression of OL markers, e.g., CLDN11, PLP1, MBP by qPCR, IHC staining, or immunoblotting. The characteristic morphology of highly complex structures (Figure 1C) and the expression of OL markers (Figure 1E) should be readily detected by Day 28.
Representative Results
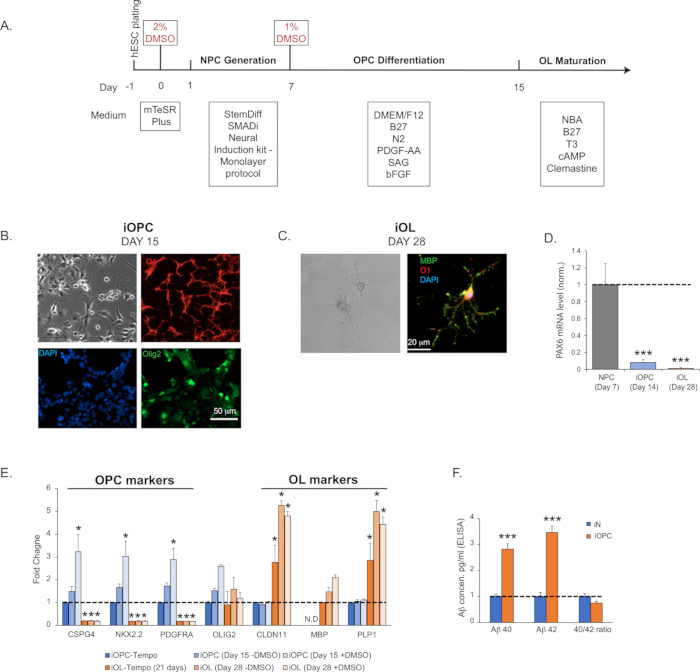
Figure 1: iOPC (induced oligodendrocyte precursor cell) generation and iOL (induced oligodendrocyte) maturation. (A) Flow diagram of iOPC and iOL generation. (B) Representative bright field and immunofluorescence images of iOPCs on Day 15. Olig2 (pan-oligodendroglia marker) is shown in green, O4 (OPC marker) in red, and DAPI in blue. The imaging revealed that >95% of iOPCs are positive for O4 and 25% for Olig2. (C) Representative bright field and immunofluorescence images of iOLs on Day 28. MBP is shown in green, O1 in red, and DAPI in blue. (D) The expression of NPC marker PAX6 diminishes dramatically in iOPCs at Day 14 and further lowers to the background in OLs at Day 28, indicating a robust NPC trans-differentiation and a high level of homogeneity in the iOPC population. (E) The time-course expression profile of common OPC and OL marker genes in cultures generated by the described protocol, without (-DMSO) or with (+DMSO) the step of DMSO incubation (steps 1.1.3 and 1.2.2), assayed at different time points. As a comparison, commercial iOPCs (see Table of Materials) were matured according to the manufacturer's instructions, and both iOPCs (iOPC-Tempo) or iOLs (iOL-Tempo) were tested for the same markers. As expected, MBP (a mature oligodendrocyte marker) was not detected (N.D.) at the early stages of differentiation in all the iOPCs tested. The DMSO significantly enhanced the efficiency of OPC differentiation and OL maturation. (F) The production and secretion of Aβ40 and Aβ42 in pure iNs (induced neurons) and iOPCs cultures, measured by commercial ELISA (enzyme-linked immunosorbent assay) kits (see Table of Materials) on supernatant obtained from pure iNs and iOPCs cultures both at Day 15 and normalized by cell numbers (both at the density of 200,000 cells per well in a 24-well plate).
Offenlegungen
The authors have nothing to disclose.
Materials
| Accutase | STEMCELL Technologies | 7920 | |
| B27 supplement | ThermoFisher | 17504044 | |
| bFGF | ThermoFisher | PHG 0266 | |
| cAMP | MilliporeSigma | A9501 | |
| Clemastine | MilliporeSigma | SML0445 | |
| DMEM/F12 medium | STEMCELL Technologies | 36254 | |
| DMSO | ThermoFisher | D12345 | |
| Fetal Bovine Serum | ScienCell | 10 | |
| H1 human ES cells | WiCell | WA01 | |
| Matrigel | Corning | 354234 | |
| mTeSR plus | STEMCELL Technologies | 5825 | |
| N2 supplement | ThermoFisher | 17502001 | |
| Neurobasal A medium | ThermoFisher | 10888-022 | |
| Non Essential Amino Acids | ThermoFisher | 11140-050 | |
| PDGF-AA | R&D Systems | 221-AA-010 | |
| pMDLg/pRRE | Addgene | 12251 | |
| Polybrene | MilliporeSigma | TR-1003-G | |
| pRSV-REV | Addgene | 12253 | |
| ROCK Inhibitor Y-27632 | STEMCELL Technologies | 72302 | |
| SAG | Tocris | 4366 | |
| STEMdiff Neural Progenitor Freezing Media | STEMCELL Technologies | 5838 | |
| STEMdiff SMADi Neural Induction Kit | STEMCELL Technologies | 8581 | |
| T3 triiodothyronine | MilliporeSigma | T6397 | |
| Tempo-iOlogo: Human iPSC-derived OPCs | Tempo BioScience | SKU102 | |
| TetO-Ng2-Puro | Addgene | 52047 | |
| VSV-G | Addgene | 12259 |

