Differentiation of a Human Neuroblastoma Cell Line into Mature Neurons
Abstract
Source: Shipley, M. M., et al. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. (2016).
This video demonstrates a technique for transforming undifferentiated SH-SY5Y neuroblastoma cells into fully differentiated mature neurons using a systematic passaging technique. This involves culturing the cells in a series of media with gradually decreasing serum levels to inhibit the proliferation of epithelial-like cells while promoting the differentiation and proliferation of neuron-like cells. Additionally, the media is supplemented with retinoic acid and specific growth factors that induce terminal differentiation of neuron-like cells into mature neurons exhibiting an extensive branched morphology.
Protocol
1. General Considerations
- See the Table of Materials/Equipment for a list of necessary reagents. Perform all steps under strict aseptic conditions.
- Use heat-inactivated fetal bovine serum (hiFBS) for all media preparations that include FBS. To heat-inactivate, warm a 50 ml aliquot of FBS at 56 °C for 30 min, inverting every 10 min (see also Table 1).
NOTE: When FBS is used without heat-inactivation, the epithelial-like phenotype progresses more quickly throughout cultures of SH-SY5Y cells. - Prior to use, allow media to warm and equilibrate in an incubator to establish a proper pH balance before every step. For example, 50 ml of media takes approximately one hour to fully equilibrate (pH 7, 37 °C, 5% CO2).
NOTE: This protocol uses a two-step splitting procedure that requires partially differentiated SH-SY5Y cells to be trypsinized and re-plated. This is a stressful process for these exceptionally fragile cells. Therefore, it is important to incubate the cells in trypsin for a minimal amount of time. This will allow for the preferential lift-off of neurons, leaving epithelial-like cells still attached to the dish. - Perform trituration of differentiated cells slowly with a 10 ml plastic pipette with the tip against the bottom of the conical tube containing the cells. Perform trituration at a slow speed, up and down no more than five times.
2. Thaw and Culture Undifferentiated SH-SY5Y Neuroblastoma Cells
- Prepare Basic Growth Media.
- Rapidly thaw frozen cells in a 37 °C water bath (approximately 2 min).
- Resuspend cells in 9 ml Basic Growth Media in a 15 ml conical tube, and then centrifuge for 2 min at 1,000 x g.
- Aspirate the supernatant while being careful not to disturb the pelleted cells, and gently resuspend cells in 10 ml Basic Growth Media.
- Plate cells onto a T-25 flask or 60 mm2 dish.
- The next day, replace media to remove dead cells.
3. Day 0: Plating Cells for Differentiation
- See Figure 1 for the differentiation schedule.
- Rinse undifferentiated cells with 1x PBS, aspirate, and then trypsinize using 1-2 ml warmed 1x 0.05% Trypsin-EDTA.
- When cells are in trypsin, incubate for approximately 3 min in an incubator.
- Quench the trypsin by adding 10 ml Basic Growth Media, rinse the sides of the flask or dish, and gently triturate 1-3 times. Transfer contents to a 15 ml conical tube.
- Centrifuge for 2 min at 1,000 x g and aspirate the media while being careful not to disturb the pellet.
- Resuspend pellet in 5 ml Basic Growth Media and triturate 1-3 times.
- Count cells using a hemocytometer, then dilute using Basic Growth Media to 50,000 cells/ml.
- Plate 2 ml of cells per 35 mm2 dish for a total of 100,000 cells per dish and place back into incubator.
4. Day 1: Change Media (Differentiation Media #1)
- Aliquot 50 ml of Differentiation Media #1 (see Table 2) and incubate in a 37 °C water bath.
- When media is warmed, allow it to equilibrate in an incubator (37 °C, 5% CO2) for at least one hr to establish a proper pH balance prior to use.
- Add Retinoic Acid (RA) (see Table 1) to warmed and equilibrated media immediately before adding media to dishes.
NOTE: Retinoic acid is light sensitive and should be stored in dark bottles at 4 °C - Gently aspirate off old media and discard.
- Add 2 ml Differentiation Media #1 with RA per 35 mm2 dish and return to incubator.
5. Day 3: Change Media (Differentiation Media #1)
- Repeat Section 4 (steps 1-5)
6. Day 5: Change Media (Differentiation Media #1)
- Repeat Section 4 (steps 1-5)
7. Day 7: Split Cells 1:1
- Add RA to warmed and equilibrated Differentiation Media #1 immediately before adding media to dishes.
- Gently aspirate off old media and discard.
- Add 200 μl warmed 0.05% 1x Trypsin EDTA per 35 mm2 dish and warm in an incubator for approximately 2-3 min or until cells are visibly lifted from the plate as observed under a microscope.
- Quench the trypsin by adding 2 ml Differentiation Media #1 with RA per 35 mm2 dish and use the media to rinse the remaining neuronal cells off the plate. Then transfer the contents to a 50 ml conical tube.
NOTE: Do not trypsinize too many dishes at once during the trypsinization steps. This helps to ensure neuronal cultures are not incubated in trypsin for too long, which can be cytotoxic. - Combine contents from up to 10 dishes in the 50 ml conical tube and gently triturate slowly up and down no more than five times with a 10 ml plastic pipette.
- Aliquot 2 ml cell suspension into fresh 35 mm2 dishes and return to incubator.
8. Day 8: Change Media (Differentiation Media #2)
- Add RA (see Table 1) to warmed and equilibrated media immediately before adding media to dishes.
- Gently aspirate off old media and discard.
- Slowly add 2 mL Differentiation Media #2 (see Table 2) with RA per 35 mm2 dish and return to the incubator. Do not allow neurons to be exposed to air for an extended period as they can dry out quickly.
9. Day 9: Prepare Extracellular Matrix (ECM) Coated Dishes
- Thaw one vial of ECM solution on ice and dilute 1:100 into cold DMEM.
- Dispense 2 ml of mixture into each 35 mm2 dish and ensure the entire base of the dish is covered.
- Place in an incubator (37 °C, 5% CO2) for 1 hr or overnight.
- Aspirate mixture and allow to air dry for approximately 1 hr in a hood. Store at room temperature for up to 2 months.
10. Day 10: Transfer Cells onto ECM Coated Plates 1:1
- Add RA (see Table 1) to warmed and equilibrated media immediately before adding media to dishes.
- Gently aspirate off media and discard.
- Add 200 μl warmed trypsin to each 35 mm2 dish and allow to incubate at room temperature for approximately 1-2 min or until neurons are visibly lifted from the dish as observed under a microscope.
NOTE: Execute this trypsinization step at room temperature to avoid over-incubating neurons with trypsin and causing damage. Neurons release from plates much faster than epithelial-like cells at this stage. - Quench the trypsin by adding 2 ml Differentiation Media #2 per 35 mm2 dish and use the media to rinse remaining neuronal cells off the plate. Then transfer contents to a 50 ml conical tube.
- Combine contents from up to 10 dishes in the 50 ml conical tube and gently triturate slowly up and down no more than five times with a 10 ml plastic pipette.
- Dispense 2 ml cell suspension into ECM-coated 35 mm2 dishes and return to the incubator.
11. Day 11: Change Media (Differentiation Media #3)
- Add RA (see Table 1) to warmed and equilibrated media immediately before adding media to dishes.
- Gently aspirate off old media and discard.
- Slowly add 2 ml Differentiation Media #3 (see Table 2) with RA per 35 mm2 dish and return to incubator. Do not allow neurons to be exposed to air for an extended period.
12. Day 14: Change Media (Differentiation Media #3)
- Repeat Section 11 (steps 1-3)
13. Day 17: Last Media Change (Differentiation Media #3)
- Repeat Section 11 (steps 1-3)
14. Day 18: Neuronal Cultures Ready to Use
- Change media to fresh Differentiation Media #3 with RA every 3 days to maintain neuron health.
NOTE: Cells should be differentiated into neurons and exhibit a neuronal phenotype. Cultures are typically stable for up to 14 days following terminal differentiation. However, the duration of neuron viability depends on the passage number of the undifferentiated cells at the start of differentiation. Higher passage numbers yield differentiated neurons with a shorter useful lifetime.
Table 1: Stock solutions and components.
| Component | Details | Stock | Instructions |
| 10 μM RA | All-trans retinoic acid | 5 mM | Resuspend 50 mg RA in 33.3 ml 95% EtOH. RA is sensitive to heat, light, and air. Keep in a dark bottle and store at 4 °C for up to 6 weeks. Use at 1:500 dilution and dilute into differentiation media immediately prior to use |
| (300.44 g/mol) | |||
| 1x B-27 | B-27 Supplement | 50x | Thaw 1-10 ml bottle and aliquot remainder into single-use 1 ml aliquots and store at -80°C. Store 10 ml bottles at -20 °C |
| 20 mM KCl | Potassium Chloride | 1 M | Add 250 ml water to 18.6 g KCl and sterile filter. Store at room temperature |
| (74.55 g/mol) | |||
| 2 mM db-cAMP | dibutyryl cyclic AMP | 1 M | Resuspend full bottle by adding 2.04 ml water to 1 g db-cAMP. Sensitive to light and moisture. Store in aliquots of 100 μl or 200 μl at either -20 °C or -80 °C |
| (491.37 g/mol) | |||
| 50 ng/ml BDNF | Brain-derived neurotrophic factor (BDNF) | – | Centrifuge vial to get powder to bottom. Resuspend 10 μg vial in 1 ml Neurobasal + 1x B27 or 5 μg vial in 0.5 ml Neurobasal + 1x B27 to get 10 μg/ml. Use at 1:200 dilution. Store working aliquots at -80 °C (ex: 250 μl) |
| hiFBS | Heat-inactivated Fetal Bovine Serum | – | Aliquot thawed FBS into 50 ml conical tubes. Heat at 56 °C in a water bath for 30 min. Remove and freeze working aliquots at -20 °C |
Table 2: Media recipes.
| Basic Growth Media | ||
| Component | Volume for 500 ml | Dilution |
| EMEM | 415 ml EMEM | |
| 15% hiFBS | 75 ml hiFBS | |
| 1x Pen/Strep | 5 ml Pen/Strep | 1:100 |
| 2 mM Glutamine | 5 ml Glutamine | 1:100 |
| *Keep for 6 weeks maximum | ||
| Differentiation Media #1 | ||
| Component | Volume for 50 ml | Dilution |
| EMEM | 48 ml EMEM | |
| 2.5% hiFBS | 1.3 ml hiFBS | |
| 1x Pen/Strep | 500 μl Pen/Strep | 1:100 |
| 2 mM Glutamine | 500 μl Glutamine | 1:100 |
| 10 μM RA | 100 μl RA (5mM stock) | 1:500 |
| *Keep for 2 weeks maximum and add RA immediately prior to use | ||
| *Do not keep extra media once RA is added – RA is unstable | ||
| Differentiation Media #2 | ||
| Component | Volume for 50 ml | Dilution |
| EMEM | 49 ml EMEM | |
| 1% hiFBS | 500 μl hiFBS | |
| 1x Pen/Strep | 500 μl Pen/Strep | 1:100 |
| 2 mM Glutamine | 500 μl Glutamine | 1:100 |
| 10 μM RA | 100 μl RA (5mM stock) | 1:500 |
| *Keep for 2 weeks maximum and add RA immediately prior to use | ||
| *Do not keep extra media once RA is added – RA is unstable | ||
| Differentiation Media #3 | ||
| Component | Volume for 50 ml | Dilution |
| Neurobasal | 47 ml Neurobasal | |
| 1x B-27 | 1 ml B-27 (50X stock) | 1:50 |
| 20 mM KCl | 1 ml KCl (1M stock) | 1:50 |
| 1x Pen/Strep | 500 μl Pen/Strep | 1:100 |
| 2 mM GlutamaxI | 500 μl GlutamaxI (100x stock) | 1:100 |
| 50 ng/ml BDNF | 250 μl BDNF stock (10 μg/ml) | 1:200 |
| 2 mM dibutyryl cyclic AMP (db-cAMP) | 100 μl db-cAMP (1M stock) | 1:500 |
| 10 μM RA | 100 μl RA (5 mM stock) | 1:500 |
| *Keep for 2 weeks maximum and add RA immediately prior to use | ||
| *Do not keep extra media once RA is added – RA is unstable | ||
Representative Results
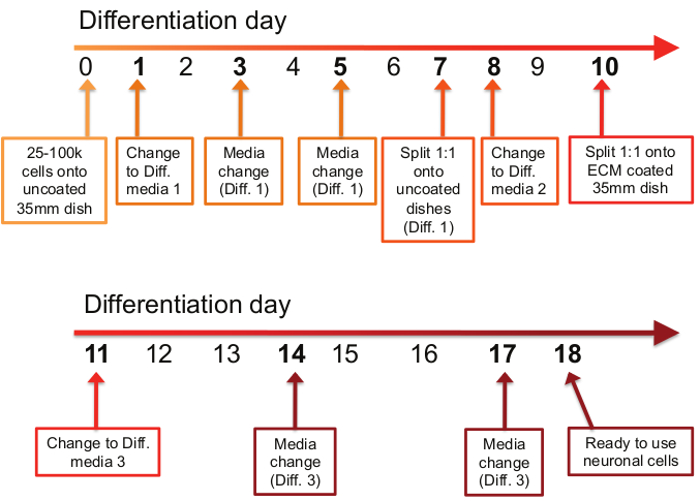
Figure 1: Timetable of differentiation procedure. The differentiation process consists of 11 steps spread out over the course of an 18 day period. On the first day of the differentiation protocol (day 0), between 25,000 and 100,000 cells are plated onto uncoated 35 mm dishes. On days 1, 3, and 5, old media is removed and Differentiation Media #1 is applied. On day 7, cells are split 1:1 onto uncoated 35mm dishes in Differentiation Media #1. On day 8, the media is changed to Differentiation Media #2, and on day 10, cells are again split 1:1, but this time onto ECM-coated 35 mm dishes in Differentiation Media #2. On days 11, 14, and 17, old media is removed and Differentiation Media #3 is applied. On day 18, differentiated neurons are ready to use for downstream applications.
Offenlegungen
The authors have nothing to disclose.
Materials
| B-27 | ThermoFisher Scientific | 17504-044 | See Table 1 for preparation |
| Brain-Derived Neurotrophic Factor (BDNF) | Sigma | SRP3014 (10 μg)/B3795 (5 μg) | See Table 1 for preparation |
| dibutyryl cyclic AMP (db-cAMP) | Sigma | D0627 | See Table 1 for preparation |
| DMSO | ATCC | 4-X | – |
| Minimum Essential Medium Eagle (EMEM) | Sigma | M5650 | – |
| Fetal Bovine Serum (FBS) | Hyclone | SH30071.03 | See Table 1 for preparation |
| GlutamaxI | Life Technologies | 35050-061 | – |
| Glutamine | Hyclone | SH30034.01 | – |
| Potassium Chloride (KCl) | Fisher Scientific | BP366-1 | See Table 1 for preparation |
| MaxGel Extracellular Matrix (ECM) solution | Sigma | E0282 | See step 11 of the protocol |
| Neurobasal | Life Technologies | 21103-049 | – |
| Penicillin/Streptomycin (Pen/Strep) | Life Technologies | 15140-122 | – |
| Retinoic acid (RA) | Sigma | R2625 | Should be stored in the dark at 4 °C because this reagent is light sensitive |
| SH-SY5Y Cells | ATCC | CRL-2266 | – |
| 0.5% Trypsin + EDTA | Life Technologies | 15400-054 | – |
| Falcon 35 mm TC dishes | Falcon (A Corning Brand) | 353001 | – |

