Establishing a Co-Culture of Dental Pulp Cells and Trigeminal Neurons for Cross-Communication
Abstract
Source: Barkley, C. et al., A Co-Culture Method to Study Neurite Outgrowth in Response to Dental Pulp Paracrine Signals. J. Vis. Exp. (2020).
This video showcases a technique for establishing a co-culture of trigeminal neurons and primary dental pulp cells using a transwell filter. In this physically separated condition, mesenchymal cells obtained from dental pulp tissue secrete paracrine signaling molecules that traverse through the pores, promoting the differentiation and migration of neurons towards the mesenchymal cells establishing a co-culture system.
Protocol
1. Plate Preparation
NOTE: Be sure the plate lid is on during all incubation and rinsing steps outside of the sterile tissue culture hood to prevent contamination during sample processing.
- Coating filters
- Dilute laminin to 10 μg/mL. Filter the diluted laminin under the hood.
- Pipette 450-500 μL of 10 μg/mL laminin into each well of a 24-well plate.
- Place a transwell filter, 3 μm porosity, in a well so that it contacts the laminin solution and leave it in a 37 °C incubator for either 2 h or overnight. Filter pores should allow some of the solution to diffuse and coat both the top and bottom of the filter. These filters should be used within 48 h or be refrigerated at 4 °C for up to 1 week.
2. Cell Plating
- Mice: Genetic alteration of dental pulp (DP) cells can be performed (but is not required) to study mesenchymal-neuronal interactions.
- DP dissection, dispersion, and plating (Figure 1)
NOTE: For DP dissection, use ultra-fine, straight-edge forceps. The ultra-fine edges will allow the user to wedge the forceps edge between the mineralized structure and DP tissue.- Harvest P5-P8 mice. At this stage, the teeth should be mineralizing, and the root is open.
- Anesthetize the neonates via hypothermia by placing them in a dish in the 4 °C refrigerator until they no longer move or respond to touch. Euthanize neonates by decapitation and in accordance with Institutional Animal Care & Use Committee (IACUC) procedures at the designated facility.
- Prepare a 3-5 mL aliquot of 0.25% trypsin-ethylenediamine tetraacetic acid (trypsin-EDTA) in a 50 mL conical tube to collect the DP from each mouse. This will facilitate the digestion of dental pulp from postnatal mice. Use more than 3 mL if digesting tissue from more than 10 postnatal mice.
- Place the head on a disposable underpad so that the mouth is toward the ceiling and the base of the neck is flat on the work surface. Use a razor blade in a sawing motion to separate the mandible from the maxilla.
- Optionally remove the tongue either with scissors or with forceps to allow easier access to the molars.
- Place the opened head in a dish atop a sterile gauze pad and place the specimen under a dissecting microscope (Figure 1D).
- Remove the alveolar bone tissue surrounding the first molars. Submandibular teeth are not fully erupted at this point. Insert forceps into alveolar opening and tease the tissue away from the tooth toward the buccal (cheek) or lingual (tongue) side of the mouth. Maxillary teeth will require full removal of the cleft around the tooth for exposure and removal.
- Gently transfer the submandibular and maxillary first molars (M1s) to a separate cell culture dish with 1x phosphate-buffered saline (PBS).
- Repeat 2.2.4-2.2.8 until all M1s are collected. Keep the dish containing the M1s on ice during the harvesting.
- Remove the Enamel Outer Organ (EOE) surrounding the outside of each M1. This can alternatively be done after step 2.2.11.
- With a set of forceps, rotate the M1 so the cusps are down and the open root is exposed. There will be an oval opening on the bottom of the tooth, and opaque DP tissue encapsulated by a thin layer of dentin and enamel.
- Using the tip of the forceps, gently loosen the DP by running one arm of the forceps around the internal circumference of the mineralized tissue. Remove DP tissue out of the mineralized structure and transfer it to a third dish containing 1x PBS. Remove the EOE if it was not already separated (Figure 1E).
- Transfer all DP tissue to 0.25% trypsin-EDTA in a 50 mL conical tube. Vortex the mixture and place in a 37 °C warm water bath for 10 min. This can be done with the same forceps or with long, vial forceps. The tissue will be difficult to disperse and will require vortexing every 3-4 min. Do NOT exceed 10 min trypsinization since the trypsin can damage cell membranes.
- Under a sterile hood, add warmed co-culture media (Table 1) to a final ratio of at least 1:1 media to trypsin to inactivate the enzyme. Larger ratios are acceptable if more tissue dispersion is desired.
- Pipette the media up and down multiple times with a 10 mL pipette to further disperse the DP in the media. Be careful to avoid large bubbles. Complete dispersion is nearly impossible due to the sticky nature of the tissue. However, it is also not necessary since cells will migrate outward from the tissue once plated.
- Transfer 1 mL of the dispersed DP to each well of a 24-well tissue culture plate (Figure 1F).
- Place the plate in an incubator at 37 °C and allow cells to attach and migrate out from the undispersed tissue for 48 h before changing media. Primary cells need to be plated at relatively high concentrations in order to reach 85-90% confluence within 1 week. If this is not achieved after 1 week, discard the plate.
- Trigeminal neuron dissection, dispersion and plating
NOTE: In this protocol, the imaging of neurite outgrowth in co-culture with DP cells was optimized using adolescent (6-week-old) B6.Cg-Tg(Thy1-YFP)16Jrs/J mice. Central and peripheral nervous systems of Thy1-YFP mice have a yellow fluorescent protein (YFP) tag whose expression begins around P6-P10 in neurons and increases exponentially throughout the nervous system during postnatal and adult life. YFP and GFP have conserved sequences that allow these nerves to be stained with anti-GFP antibodies, resulting in a pan-neuronal stain. Ultimately, these mice allow for better visualization and quantification of the neurons used and grown in cell culture.- Euthanize adolescent mice with carbon dioxide followed by cervical dislocation.
- Decapitate the mice and remove the skin from the skull. Be sure to include equivalent numbers of males and females.
- Insert the tip of a pair of micro-dissecting scissors into the base of the skull. Cut along the sagittal suture of the skull (Figure 1A).
- Make four small horizontal cuts: two along the coronal sutures by the ears, and two along the lambdoid sutures at the base of the skull. This should create two flaps of bone.
- Use the forceps to peel back the two flaps of bone. This should reveal the brain.
- Remove the brain. Transfer the head to a tissue culture dish with 1x PBS and put under the microscope.
- Locate the trigeminal (TG) ganglia, which is easily visible in rodents13, housed in the dura mater between the brain and bone of the maxillary process (Figure 1B).
- Cut the three branches that travel to the eyes, maxilla and mandible and transfer the ganglia to cold 1x PBS using straight-edge fine forceps. Keep the dish containing the TG ganglia on ice during harvesting.
- Once all TG bundles are harvested, transfer ganglia to a 50 mL conical tube containing 5 mg/mL sterile-filtered collagenase type II using vial forceps.
- Vortex the collagenase with the TG bundle and place the tube in a 37 °C water bath for 25-30 min. During this time, take the conical tube out of the water bath, vortex, and return to the bath every 5-10 min.
- Centrifuge the collagenase-TG neuron solution for 2 min at 643 x g.
- Under a tissue culture hood, gently aspirate the collagenase with a micropipette.
- Add 5 mL of 1% sterile-filtered trypsin type II and vortex. Place the conical tube in a 37 °C water bath for 5 min.
- Centrifuge the trypsin-TG mix for 5 min at 643 x g. Remove the top portion of trypsin with a micropipette, so that the TG neurons are not removed. There will still be liquid in the tube.
- Add enough media to deactivate the remaining trypsin (at a 1:1 or lower ratio of trypsin to media).
- Count the number of cells and dilute the solution to 200,000 cells/mL (250 μL of cell-containing 50,000 cells).
- Place coated transwell filters from section 1.2 into wells with DP.
- Dilute the cell-containing solution so that there are 200,000 cells/mL. Pipette 250 μL onto the transwell filter, and culture the cells at 37 °C overnight (Figure 1F).
- The next day, replace the media with 1 mL of Co-culture media with 1 μM uridine and 15 μM 5'-fluoro-2 deoxyuridine to stop the over-proliferation of mesenchymal cells that may prevent neurite outgrowth. Optional: Add growth factors or inhibitors in this media if attempting further manipulation.
Table 1: Co-culture media.
| Component | Volume | Concentration |
| MEM α | 440 mL | |
| Heat inactivated fetal bovine serume | 50 mL | 10% |
| 100x L-glutamine | 5 mL | 1x |
| Penicillin-streptomycin 100 x | 5 mL | 1x |
| Change media on day 2 with mitotic inhibitors at these final concentrations | ||
| Uridine | 1 μM | |
| 5'-Fluor-2'deoxyuridine | 15 μM | |
Representative Results
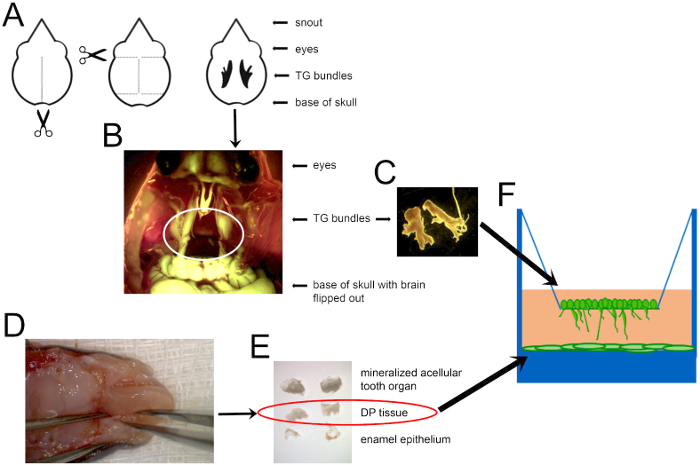
Figure 1: A schematic of the mouse dissection to obtain cells for co-culture. (A) A diagram of where to cut to open the mouse skull and locate TG nerves, shown in black in the last depiction. Scissors indicate where to insert the scissor tips to cut along the dotted lines. (B) A combined darkfield and GFP image showing Thy1-YFP+ TG nerves circled in white. (C) Dissected TG ganglia can then be dispersed and cultured, as shown in F. (D) The mandible of a P7 mouse, with forceps holding the mandible on the left and alveolar bone ridges containing unerupted teeth on each side of the tongue. (E) DP tissue (circled) extracted from the mineralized structure (top), and the enamel outer epithelium (bottom) that was removed to disperse and plate in a tissue culture-treated plate, as shown in F. Images are not shown to scale. DP cells were dispersed and grown to confluence before adding TG neurons.
Offenlegungen
The authors have nothing to disclose.
Materials
| 5-Fluoro-2'-deoxyuridine | Sigma-Aldrich | F0503 | Used as a mitotic Inhibitor at 15 μM concentration in co-culture media, Day 2 |
| 24 Well Cell Culture Plate | Corning | 3524 | Co-culture plate |
| B6;129- Tgfbr2tm1Karl/J | The Jackson Laboratory | 12603 | Tgfbr2f/f mouse model used for dental pulp cells in optimized protocol |
| B6.Cg-Tg(Thy1-YFP)16Jrs/J | The Jackson Laboratory | 3709 | Thy1-YFP mouse model genotype used for trigeminal neurons |
| Collagenase Type II | Millipore | 234155-100MG | Used to disperse trigeminal neurons |
| Fetal Bovine Serum | Gibco | 10437 | Additive to co-culture media |
| Fine forceps | Fine Science Tools | 11413-11 | Fine forceps for TG dissection |
| Laminin | Sigma-Aldrich | L2020 | Coats the transwell inserts at final concentration of 10 μg/ml, stock solution is assumed at 1.5 mg/ml |
| L-Glutamine | Gibco | 25030081 | Additive to co-culture media |
| Micro-dissecting scissors | Sigma-Aldrich | S3146-1EA | Dissection scissors to open skull |
| Minimal Essential Medium a | Gibco | 12571063 | Co-culture media base |
| Penicillin-Streptomycin | Gibco | 15070063 | Antibiotic additive to co-culture media |
| Phosphatase Inhibitor | Sigma-Aldrich | 04 906 837 001 | Additive to RIPA Buffer for extracting protein from dental pulp cells post co-culture |
| ThinCert Cell Culture Insert | Greiner Bio-One | 662631 | Transwell inserts for trigeminal neurons in co-culture assays |
| Trypsin-EDTA (0.25%) | Gibco | 25200056 | Used fto disperse dental pulp cells |
| Trypsin Type II | Sigma-Aldrich | T-7409 | Used to disperse trigeminal neurons |
| Ultra Fine Forceps | Fine Science Tools | 11370-40 | Ultra fine forceps for dissection |
| Uridine | Sigma-Aldrich | U3750 | Used as a mitotic Inhibitor at 1 μM concentration in co-culture media, Day 2 |
| Vial forceps | Fine Science Tools | 110006-15 | Long forceps for tissue transfer to conicals |

