Stimulating Neuronal Cell Differentiation Using Gold Nanorods
Abstract
Source: Paviolo., et al. Gold nanorod-assisted optical stimulation of neuronal cells. J. Vis. Exp. (2015).
This video demonstrates a technique to promote neuronal differentiation by exposing nerve cells to gold nanorods and laser light, which forms cell projections supported by the βIII-tubulin. Staining and observing tubulin and the cell nucleus with epifluorescence microscopy reveals the cell outgrowths, indicating the nanorods' efficacy in neuronal differentiation.
Protocol
1. NG108-15 Neuronal Cell Line Culture and Differentiation
- For the cell culture medium, prepare 500 ml of sterile Dulbecco's modified Eagle medium (DMEM) containing 10% (w/v) fetal calf serum (FCS), 1% (w/v) L-glutamine, 1% (w/v) penicillin/streptomycin and 0.5% (w/v) amphotericin B.
Note: Supplements can be aliquoted, stored at -20 °C, and added to the media on the day required. Cell culture medium can be refrigerated in a sterile condition for a maximum of 1 month. - For the cell differentiation medium, prepare 50 ml of sterile DMEM containing 1% (w/v) L-glutamine, 1% (w/v) penicillin/streptomycin, and 0.5% (w/v) amphotericin B.
- Grow NG108-15 neuronal cells in 10 ml of cell culture medium in T75 flasks made of polystyrene in an incubator with humidified atmosphere (5% CO2 at 37 °C). Normally, seed 1.5-2 × 105 cells in each flask to be ready in 3-4 days. Change the cell culture medium every two days.
Note: To prevent genetic drifts or variation, do not use cells older than passage 21 for experiments. - When 70-80% confluent in culture, change the medium with a warm fresh cell culture medium. Mechanically detach the cells by gently knocking the bottom of the confluent flask. Do not use trypsin.
- Centrifuge the cell suspension for 5 min at 600 x g and re-suspend the cell pellet in 2 ml of warm cell differentiation medium.
- Seed 2 × 104 cells/cm2 in a tissue culture polystyrene 96 well plate with 200 µl of cell differentiation medium. Incubate the experiment for 1 day at 5% CO2 / 37 °C.
- Add between 3.2 × 109 – 4.2 × 1010 particles/ml of gold nanorod (Au NR) solution on day 2 and incubate it for an additional 24 hr. Do not add the particles for the control experiments.
Note: As an alternative control, gold nanoparticles (Au NPs) with a well-differentiated peak absorption wavelength can be used for comparison purposes, For a more consistent cell behavior, keep the cell density constant and do not modify the well surface prior to cell seeding.
2. Neurite Outgrowth Enhancement
- Couple the laser with a single-mode optical fiber (numerical aperture = 0.13) and terminate it with a fiber connector (FC connectors are convenient and commonly available). Measure the output laser power with a standard power meter. To obtain the most effective results, match the peak wavelength of the laser to the plasmon resonance peak of the NRs.
- On day 3 following NR incubation, fix the FC connector to the well. Irradiate samples and controls at RT for 1 min in continuous wave for different laser powers. Allow the culture to proceed for 3 additional days at 5% CO2 / 37 °C. Repeat the laser irradiation for a minimum of 3 independent measurements.
Note: Different irradiation times and pulse frequencies may be selected depending on the application. - Characterize the laser in terms of beam diameter and laser irradiance (W∙cm-2).
- For a standard single-mode fiber, use NA = n∙sinθ, where n is the refractive index of the medium in use and θ is the half-angle of the cone of light exiting the fiber (see Figure 1A). From trigonometry, r = tanθ∙d, where r is the beam radius and d is the distance between the fiber and the sample. The FC connector matches the diameter of the well, therefore illuminating the sample at a distance d = 2.70 ± 0.20 mm. Light exits the fiber in water (n = 1.33), giving r = tan(sin-1(NA/n))∙d.
- Using the latter equation, calculate the laser beam radius, the corresponding beam area, and the average laser irradiance (laser power divided by beam area) at the target (see the example graph in Figure 1B). These values represent the average irradiance over the illuminated area.
- Evaluate the errors for the measurements with the general theory of error propagation.
Note: The distance between the FC connector and the sample can be measured by photographs of the experimental arrangement and post-processing of the image using appropriate software (e.g. ImageJ).
- At day 5, remove the cell differentiation medium from the experiments and fix the samples with 3.7% (v/v) formaldehyde solution for 10 min, then permeabilize the cells with 0.1% (v/v) Triton X-100 for 20 min.
- Add 3% (w/v) bovine serum albumin (BSA) to the samples for 60 min to block the unreacted protein binding sites. Label the samples for anti-βIII-tubulin overnight (5 µg/ml in PBS supplemented with 1% of BSA) at 4 °C.
- Incubate the cells for 90 min in the dark with an appropriate secondary antibody (e.g. TRITC(Tetramethylrhodamine)-conjugated anti-mouse IgG antibody) using a concentration of 0.4-2 µg/ml in 1% BSA in PBS. Label the cell nuclei with DAPI (4′,6-diamidino-2-phenylindole) (0.1 µg/ml in de-ionized water) for 10 min.
Note: Antibody conjugation and concentration might vary according to the company protocol. Wash samples with PBS twice for 5 min after each staining stage. - Image samples by epifluorescence or confocal microscopy using at least a 20× objective. Choose the microscope filters according to the secondary antibody. Select a DAPI filter (λEX = 358 nm; λEM = 488 nm) to visualize the cell nuclei.
- Analyze the pictures by assessing: i) the maximum neurite length (record the length from the tip of the neurite to the beginning of the cell body), ii) the number of neurites per neuronal cell (sum up all of the neurites per cell) and iii) the percentage of cells with neurites (divide the total number of cells expressing βIII-tubulin by the total number of cells with a positively stained nucleus)
Representative Results
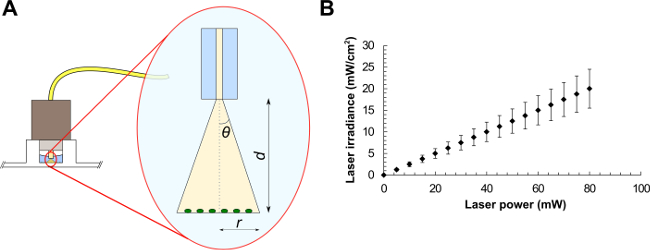
Figure 1. Optical fiber experimental setup (A) and average laser irradiances as a function of the laser power for a laser beam of area equals to 0.4 mm2 (B). Beam parameters are (A): the half-angle of the maximum cone of light exiting the fiber (θ), the beam radius (r) and the distance between the optical fiber and the sample (d).
Offenlegungen
The authors have nothing to disclose.
Materials
| Au NR | Sigma Aldrich | 716812 | |
| NG108-15 | Sigma Aldrich | 8811230 | |
| DMEM | Sigma Aldrich | D6546 | |
| FCS | Life Technologies | 10100147 | |
| L-glutamine | Sigma Aldrich | G7513 | |
| Penicillin/streptomycin | Life Technologies | 15140122 | |
| Amphotericin B | Life Technologies | 15290018 | |
| Formaldehyde | Sigma Aldrich | F8775 | |
| Triton X-100 | BDH | T8532 | |
| BSA | Sigma Aldrich | A2058 | |
| Anti-βIII-tubulin | Promega | G7121 | |
| TRITC-conjugated anti-mouse IgG antibody | Sigma Aldrich | T5393 | |
| DAPI | Invitrogen | D1306 | |
| UV-Vis spectrometer | Varian Medical Systems Inc. | Cary 50 Bio | |
| Mini centrifuge | Eppendorf | Mini Spin | |
| Sonic bath | Unisonics Australia | FPX 10D | |
| Cell culture incubator | Kendro | Hera Cell 150 | |
| Cell culture centrifuge | Hettich | Rotofix 32A | |
| Laser diode | Optotech | 780 nm single mode fibre – coupled LD | |
| Optical fiber | Thorlabs | 780 HP | |
| Power meter | Coherent | Laser Check | |
| ImageJ | http://rsb.info.nih.gov/ij/index.html | ||
| Epifluorescent microscope | Axon Instruments | ImageX-press 5000A | |
| PBS | |||
| Polystyrene 96 well plate | |||
| CO2 | |||
| t75 flasks |

