Repetitive Tumor Challenge Assay: An In Vitro Culture Assay to Evaluate Chimeric Antigen Receptor (CAR) T Cells for Repetitive Tumor Killing Potential
Abstract
Source: Wang, D. et al. In Vitro Tumor Cell Rechallenge for Predictive Evaluation of Chimeric Antigen Receptor T cell Antitumor Function. J. Vis. Exp. (2019)
In this video, we demonstrate the repetitive tumor challenge assay to evaluate the CAR T cells’ repetitive tumor killing potential during high tumor cell loads. Further, the CAR T cells can be analyzed using flow cytometry to determine the different subpopulations of CAR T cells in the sample.
Protocol
1. Media Preparation
- Prepare neural stem cell media for culturing primary GBM cell lines: DMEM:F12, 1:50 B27, 5 µg/mL heparin, and 2 mmol/L L-glutamine; supplemented with 20 ng/mL epidermal growth factor (EGF) and 20 ng/mL basic fibroblast growth factor (FGF) twice a week (see Table of Materials).
- Prepare T cell media: X-VIVO 15 containing 10% fetal calf serum (FCS); supplemented with 70 IU/mL rhIL-2 and 0.5 ng/mL rhIL-15 every 48 h (see Table of Materials).
- Prepare co-culture media: take neural stem cell media without EGF and FGF supplement, and add 10% FCS.
- Prepare FACS staining solution (FSS): HBSS, 2% FCS, NaN3 (0.5 g/500 mL).
2. Preparation of GBM Tumor Cells
- Harvest low-passage GBM tumor spheres (TSs) by centrifugation at 300 x g for 4 min and discard supernatant.
NOTE: GBM tumor spheres (TSs) are generated from resected tumors as described previously, and maintained in neural stem cell media, in incubators with 5% CO2 at 37 °C. - Pre-warm co-culture media in a 37 °C water bath.
- Add 1 mL of cold accutase to GBM TSs, dissociate TSs by pipetting for 30–60 s, and stop dissociation by adding 5 mL of warm co-culture media.
NOTE: GBM TSs should not be kept on accutase for more than 5 min. - Harvest GBM cells by centrifugation at 300 x g for 4 min, discard supernatant, and resuspend cells in 2 mL of co-culture media.
- Determine cell concentration using a cell viability counter.
NOTE: Cell viability must be >70%.
3. Preparation of CAR T Cells
- Less than 24 h before the assay, take 100 µL of cultured CAR T cells into a flow cytometry tube and add 2 mL of FSS.
NOTE: CAR T cells were generated and cultured in T cell media. - Centrifugation at 300 x g for 4 min and discard supernatant.
- Add 2 mL of FSS to wash the cells, centrifuge at 300 x g for 4 min, and discard supernatant.
- Stain the cells with appropriate antibody to indicate CAR expression at 4 °C for 30 min.
NOTE: For example, if an IL13Rα2-targeted CAR is used, then stain with anti-IL13 antibody. - Wash twice with 2 mL of FSS and analyze CAR expression using a flow cytometer.
- Determine the CAR% on T cells using the gating strategy shown in Figure 1B.
- At the day of co-culture, harvest all CAR T cells by centrifugation at 300 x g for 4 min, discard supernatant, and resuspend cells in 2 mL of co-culture media.
- Determine cell concentration using a cell viability counter.
NOTE: Cell viability must be >70%.
4. Set up tumor — T Cell Co-culture
- Dilute tumor cells to a concentration of 0.16 million/mL with co-culture media.
- Based on CAR%, dilute CAR T cells to a concentration of 0.04 million CAR+ cells/mL with co-culture media.
NOTE: For example, if the CAR is 50% and T cell concentration is 0.4 million/mL, then make a 1:5 dilution to get the final concentration of 0.04 million CAR+ cells/mL. - Pipette 100 µL of diluted tumor cells into each well of a 96-well flat-bottom tissue culture plate.
- Pipette 100 µL of diluted CAR T cells into each well that contains tumor cells and mix well.
NOTE: Each tumor-T cell co-culture will be analyzed at 4 time points. 4–6 replicates might be required at every time point based on different analysis, but <3 replicates per time point is not recommended; see Table 1 for a representative platemap. - Maintain the plate in a 37 °C, 5% CO2 incubator.
5. Tumor Cell Rechallenge
NOTE: Rechallenge takes place at 2, 4 and 6 days post the initial co-culture setup (Figure 1A).
- Harvest and dissociate GBM TSs as described above (steps 2.1–2.6).
- Resuspend GBM cells at a concentration of 0.64 million/mL.
- Determine the co-culture wells that need rechallenge (see Table 1) and carefully remove 50 µL media from the top of each well.
- Add 50 µL of GBM cell suspension into each well and mix well, then put the plate back into a 37 °C, 5% CO2 incubator.
6. Harvest Samples and Flow Cytometric Analysis
NOTE: Samples will be harvested at 1, 3, 5 and 7 days post the initial co-culture setup, with 1, 2, 3 and 4 rounds of tumor challenge, respectively.
- Pre-warm 0.05% trypsin-EDTA solution in 37 °C waterbath.
- Determine the wells that need harvesting and transfer the media into a new round-bottom 96-well plate.
- Pipette 50 µL of trypsin-EDTA into the wells to digest remaining tumor cells at 37 °C for 5 min.
- Under a microscope, confirm the cells have detached from the bottom.
- Pipette around the well bottom to resuspend detached cells, then transfer trypsin-EDTA containing detached cells to the corresponding wells of the round-bottom 96-well plate.
- Centrifuge the round-bottom 96-well plate at 300 x g, 4 °C for 4 min, then discard supernatant.
- Add 200 µL/well of FSS to wash the cells, centrifuge at 300 x g, 4 °C for 4 min, then discard supernatant.
- Resuspend cells in 100 µL/well FSS containing antibodies (see Table of Materials), and stain cells at 4 °C for 30 min.
- Add 100 µL/well FSS to cells, centrifuge at 300 x g, 4 °C for 4 min, then discard supernatant.
- Add 200 µL/well of FSS to wash the cells, centrifuge at 300 x g, 4 °C for 4 min, then discard supernatant.
- Resuspend cells with 100–200 µL/well of FSS with 500 ng/mL DAPI, then analyze samples by flow cytometry.
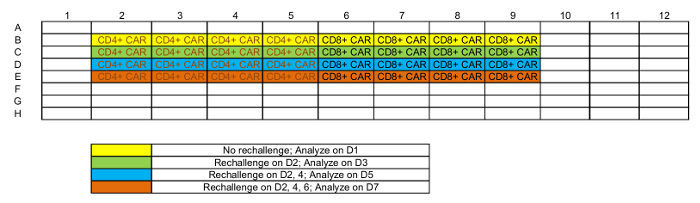
Table 1: Representative plate map of rechallenge setup.
Representative Results
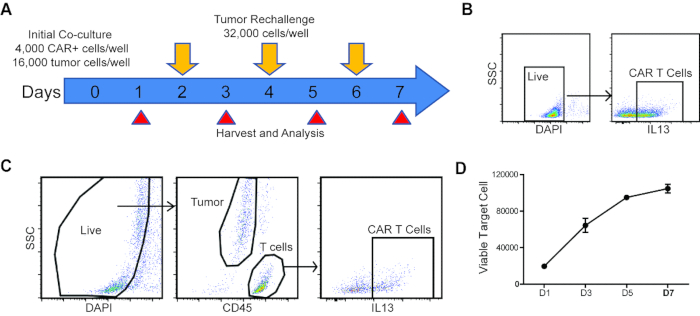
Figure 1: Schema and analysis strategy of repetitive challenge assay. (A) Schema and timeline of repetitive tumor challenge assay. For each well, CAR T cells were first co-cultured with PBT030-2 GBM cells (4,000 CAR+ cells, 16,000 tumor cells) and re-challenged with 32,000 tumor cells every other day (D2, D4 and D6). Analysis of tumor cell and CAR T cell number, as well as CAR T cell phenotype is carried out at D1, D3, D5 and D7. (B) Gating strategy to determine CAR% in T cells before setting up the co-culture. (C) Gating strategy of live cells, tumor cells and CAR T cells from the repetitive challenge assay. (D) Tumor cells number at different times of the rechallenge assay, co-cultured with untransduced T cells. Error bars = ±SEM.
Offenlegungen
The authors have nothing to disclose.
Materials
| 0.05% Trypsin/0.53mM EDTA in HBSS without Calcium and Magnesium | Corning | MT25051CI | |
| 1 M Hepes | Irvine Scientific | 9319 | |
| 200 mM L-Glutamine | Cambrex Bio Science | 17-605E | |
| 4',6-diamidino-2-phenylindole, dihydrochloride (DAPI) – FluoroPure grade | Invitrogen | D21490 | |
| Accutase | Innovative Cell Technologies | AT104 | |
| Aldesleukin Proleukin (rhIL-2) | Novartis Oncology | NDC 0078-0495-61 | |
| Anti-CD137, PE | BD Biosciences | 555956 | 4B4-1 |
| Anti-CD19, PE-Cy7 | BD Biosciences | 557835 | SJ25C1 |
| Anti-CD3, PERCP | BD Biosciences | 340663 | SK7 |
| Anti-CD4, FITC | BD Biosciences | 340133 | SK3 |
| Anti-CD45, PERCP | BD Biosciences | 340665 | 2D1 |
| Anti-CD45RO, PE | BD Biosciences | 561137 | UCHL1 |
| Anti-CD62L, APC | BD Biosciences | 559772 | DREG-56 |
| Anti-CD69, APC | BD Biosciences | 340560 | L78 |
| Anti-CD8, APC-Cy7 | BD Biosciences | 348793 | SK1 |
| Anti-IL-13, PE | BD Biosciences | 340508 | JES10-5A2 |
| Anti-LAG-3, PE | eBiosciences | 12-2239-41 | 3DS223H |
| Anti-PD-1, APC-Cy7 | BioLegend | 329921 | EH12.2H7 |
| Anti-TIM-3, APC | eBiosciences | 17-3109-42 | F38-2E2 |
| B-27 Serum-Free Supplement (50x) | Invitrogen | 17504-044 | |
| Corning 96 Well Clear Flat Bottom Polystyrene TC-Treated Microplates, Individually Wrapped, with Lid, Sterile (Product #3596) | Corning Life Sciences | 3596 | |
| Defined fetal bovine serum,HI IR | Hyclone Labs | SH30070.03IH | |
| DMEM F-12 50:50 (1x) without Glutamine | MediaTech, Inc. | 15-090-CV | |
| DMEM High Gluc w/o L-Glu, Na Pyr 1 L | Invitrogen | 11960051 | |
| DMEM-Ham's F12 50:50 Mixture with L-glutamine and 15 mM HEPES | Fisher Scientific | MT10092CV | |
| HBSS | Irvine Scientific | 9224 | |
| Heat-Inactivated FCS | Hyclone | SH30070.03 | |
| Heparin Sodium(1,000 U/mL) | American Pharmaceutical | 401811B | |
| PBS 1x W/CA & MG | Irvine Scientific | 9236 | |
| PBS with 1 mM EDTA (No Ca2+ or Mg2+) | VWR Scientific Products | PB15009A | |
| rhIL-15 Working Dilution (10 ng/µL) | CellGenix, US Operations | tF0297 | |
| Sodium Azide (NaN3) | Sigma | S8032 | |
| Recombinant human EGF | R&D Systems | 236-EG-200 | |
| Recombinant human FGF | R&D Systems | 233-FB |

