Comprehensive Compositional Analysis of Plant Cell Walls (Lignocellulosic biomass) Part II: Carbohydrates
Summary
Plant biomass is a major carbon-neutral renewable resource that could be used for the production of biofuels. Plant biomass consists mainly of cell walls, a structurally complex composite material termed lignocellulosics. Here we describe a protocol for a comprehensive analysis of the content and composition of wall derived carbohydrates.
Abstract
Protocol
1. Cell wall isolation
- grind roughly 60-70mg of air- or freeze dried plant material with 5.5 mm stainless steel balls in a 2ml sarstedt screw cap tube using a retschmill (1 min, 25 Hz). An alternative, the use of a high-throughput grinding and dispensing robot termed iWall is described in Part I3.
- remove the steel balls before continuing with the cell wall isolation procedure
The detailed protocol of the preparation of cell wall material is shown in Part I3. For completeness here the written steps of the protocol. - add 1.5 ml of 70% aqueous ethanol, and vortex thoroughly
- centrifuge at 10,000 rpm for 10 min to pellet the alcohol insoluble residue
- aspirate or decant the supernatant
- add 1.5 ml of chloroform/methanol (1:1 v/v) solution to the residue and shake tube thoroughly to resuspend the pellet
- centrifuge at 10,000 rpm for 10 min and aspirate or decant the supernatant
- resuspend pellet in 500 ul of acetone
- evaporate the solvent with a stream of air at 35°C until dry
If needed dried samples can be stored at room-temperature until further processing. - To initiate the removal of starch from the sample re-suspend the pellet in 1.5 ml of a 0.1 M sodiumacetate buffer pH 5.0.
- cap the sarstedt tubes and heat for 20 min. at 80°C in a heating block.
- cool the suspension on ice
- add the following agents to the pellet: 35 μl of 0.01% Sodiumazide (NaN3), 35 μl Amylase (50 μg/1mL H2O; from Bacillus species, SIGMA); 17 μl Pullulanase (18.7 units from bacillus acidopullulyticus; SIGMA). Cap the tube and vortex thoroughly.
- The suspension is incubated over night at 37°C in the shaker. Orienting the tubes horizontally aides improved mixing.
- heat suspension at 100°C for 10 min in a heating block to terminate digestion.
- centrifuge (10,000 rpm, 10 min) and discard supernatant containing solubilized starch
- wash the remaining pellet three times by adding 1.5 ml water, vortexing, centriguation, and decanting of the washing water.
- resuspend pellet in 500 ul of acetone
- evaporate the solvent with a stream of air at 35°C until dry. It may be necessary also to break up the material in the tube with a spatula for better drying.
The dried material presents isolated cell wall (lignocellulosics). If needed dried samples can be stored at room-temperature until further processing.
2. Matrix Polysaccharide composition
This method is essentially a modification of the method published by Albersheim 1.
- To determine the monosaccharide composition of the wall material weigh 2 mg of cell wall material into 2ml starstedt tubes either by hand or in a highthroughput fashion using the iWall, a robotic grinding and weighing robot.
- Add 20 uL of an Inositol solution (5mg/ml) as an internal standard. For a 2 mg cell wall sample we recommend to add 100 ug.
- rinse tube walls with 250 ul of acetone to collect the cell wall material on the bottom of the tube, and evaporate the acetone very gentle under airflow.
- For the weak acid hydrolysis add 250 ul of 2M trifluoroacetic acid (TFA) to each sample. Add TFA carefully to ensure no material is splashed up onto the tube walls.
- cap tightly and incubate for 90 min at 121°C in a heating block.
- cool the heating blocks and samples on ice.
- centrifuge the tubes at 10,000 rpm for 10 min.
- transfer 100 ul of the acidic supernatant containing the matrix polysaccharide derived monosaccharide to a glass screw cap vials making sure not to disturb the pellet material. The pellet can be used for the crystalline cellulose assay below (see 3.)
- evaporate the TFA in the glass tube under a gentle stream of air in an evaporation device.
- add 300 μl 2-Propanol, vortex and evaporate at 25°C (repeat for a total of three times)
- The first step of the alditol acetate derivatization procedure is to perform a reduction of the monosaccharides to their corresponding alditols. For this purpose add 200 μl of a sodium borohydride solution to each dried sample. Prepare a fresh solution each time using 10mg of Sodium borohydride per 1ml of 1M Ammonium hydroxide.
- leave glass vial at room temperature for 1.5 hours
- neutralize the solution by adding 150 ul of glacial acetic acid
- vortex and evaporate at 25°C .
- add 250 μl Acetic acid/Methanol (1:9, v/v), vortex and evaporate at 25˚C
- add 250 μl Methanol, and evaporate under stream of air (repeat for a total of three times)
- For the acetylation of the alditols, add 50 μl of Acetic anhydride and 50 μl of Pyridine, vortex and incubate for 20 min at 121°C in a heating block.
- cool samples in the block down with ice while wait for temp decrease to approximately room temperature.
- evaporate the reagents under a gentle stream of air at room temperature. Be careful: alditol acetates are highly volatile.
- add 200 μl Toluene and evaporate under air (x3)
- In the final steps the alditol acetates are extracted. First, add 500 ul of ethyl acetate and swirl lightly.
- add 2 ml of water, cap tubes and vortex.
- centrifuge tubes at 2,000 RPM for 5 min to obtain clear separate layers (ethyl acetate on top, water on bottom)
- pipette 50 ul of the ethyl acetate layer into GC/MS vials with inserts.
- dilute by adding 100 ul of acetone to the GC-vial and cap. The sample volume and dilution volumes can be adjusted to avoid overloading the GC/MS if the sample concentration is too high.
The GC-vial can be stored at 4°C, if the GC/MS analysis does not immediately proceed - The samples are injected into a GC that is equipped with a quadrupole MS, but a flame ionization detector is also suitable. A Supelco SP-2380 (30mm X 0.25mm x 0.25 μm film thickness) column is used with a 4min solvent delay and a flow rate of 1.5ml/min. Injected samples are subjected to the following temperature program: Initial hold at 160°C for 2 min; a 20°C/min ramp to 200°C and hold for 5 min; a 20°C/min ramp to 245°C and hold 12 min; spike to 270°C and hold for 5 min before cooling to the initial temperature of 160°C 2.26.) Peaks are identified by mass profiles and/or retention times of standards. Monosaccharides are quantified based on standard curves.
3. Crystalline Cellulose Content
This method is essentially described by Updegraf8. There are a number of starting materials for this procedure: Isolated cell wall material (see 1) or wall material that has already been treated with 2M TFA (see 2.8) either the remaining pellet immediately after the acid treatment (see 2.8) or a TFA pellet, that has been washed with 2-propanol and dried.
- Add to the TFA pellet in the screw capped glass tube 1 ml of Updegraff reagent (Acetic acid: nitric acid: water, 8:1:2 v/v).
- Cap tube tightly, vortex, and heat in a heating block at 100°C for 30 min. As a result of this treatment only crystalline cellulose remains insoluble in the pellet.
- Cool samples in the block on ice to room-temperature or cooler
- centrifuge samples at 10,000 rpm for 15 min
- Discard supernatant ensuring that the pellet is not disturbed and no material from the pellet is removed. For this purpose leave approx. 150 ul of supernatant in the tube.
- add 1.5 ml of water, shake, centrifuge, and discard supernatant as done above
- repeat washing procedure 3 additional times using 1.5 ml of acetone
- Air dry pellet very gently with air, or let dry on bench overnight
- the pellet (crystalline cellulose) is now completely hydrolyzed into glucose by what is called a Saeman hydrolysis. For this purpose add 175 μl 72% Sulfuric acid to the sarstedt tube
- incubate at room temperature for 30 min, vortex and incubate for another 15 min
- add 825 μl water and vortex
- centrifuge samples at 10,000 rpm for 5 min. There might be some brown insoluble material, lignin, remaining in the tube.
- The glucose content of the supernatant is assayed using the colorimetric anthrone assay. This assay is performed in a 96 well polystyrene microtiter plate.
- For the standard curve use a 1mg/ml glucose stock (stored at 0°C) and create duplicate 0, 2, 4, 6, 8, and 10 ug standards by pipetting 0, 2, 4, 6, 8, and 10 ul into separate appropriate well. Fill each well up to 100 ul with water.
- add 10 μl of each sample supernatant and 90 ml of water into separate cells but on the same microtiter plate as the standard.
- add 200 μl of freshly prepared Anthrone Reagent (Anthrone dissolved in concentrated sulphuric acid , 2 mg anthrone/ ml sulphuric acid)
- heat plate for 30 min at 80°C in an oven (aluminum heat spreader). Glucose containing samples turn from yellow into blue-green.
- let the plate cool to room-temperature and shake thoroughly.
- read absorption of plate at 625 mm using a microtiter plate reader.
- Glucose (and hence crystalline cellulose content) is calculated based on the absorbance compared to the standard curve established on the same plate.
4. Representative Results
An example of a wall analysis is presented in Figure 2. In this case poplar stem (wood) was analyzed by the various procedures outlined in the protocol section. The matrix polysaccharide composition is highlighted by an example chromatogram identifying the typical sugar present in plant cell walls, fucose, rhamnose, xylose, arabinose, galactose, mannose and glucose (and the internal standard inositol). The main hemicellulosic component of poplar is xylan as demonstrated by the high xylose content. However, the abundance of these sugars will vary depending on the feedstock used4. The glucose in this analysis is derived from the hemicellulose xyloglucan and amorphous cellulose. Due to the analysis the data can be presented as mol% or ug/ mg wall material (or dry weight). The content of crystalline cellulose is self-explanatory, one can expect values of between 20-50% of the wall dry weight. Based on the results presented here and in Part I3 the lignocellulosic composition of poplar wood is 21% lignin, 30% hemicelluloses, and 41% crystalline cellulose. The remainder would be ash.
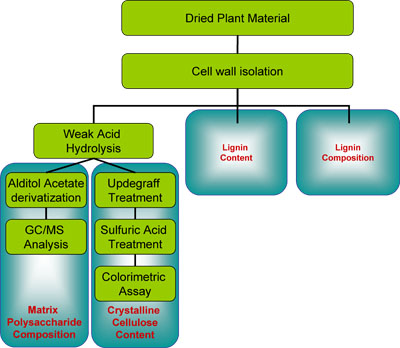
Figure 1: Overview of lignocellulosic analysis. Cell walls (lignocellulosics) are isolated from crude dried plant material. The wall material is then weighted into aliquots and subdivided for the various assays. Matrix polysaccharide composition is established after treating the wall material with a weak acid (2M TFA), derivatizing the resulting solubilized monosaccharides to their alditol acetates, and analysis by GC-MS. The residue of the weak acid treatment is washed with the so-called Updegraff reagent leaving only insoluble crystlline cellulose behind. The cellulose is solubilized by sulfuric acid and quantified by a colorimetric assay determining the glucose content. In parallel, the content and composition of lignin can be determined as described in Part I3.
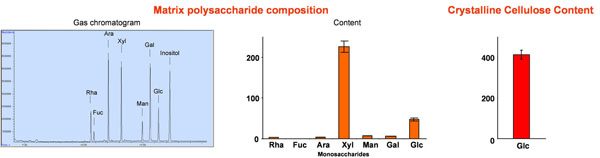
Figure 2: Comprehensive lignocellulosic analysis of poplar wood. Wood chips from poplar (Populus tremoloides) were subjected to the described protocol.
Upper left: Matrix polysaccharide composition; Fuc fucose; Rha rhamnose; Ara arabinose; Xyl xylose; Man mannose; Gal galactose; Glc glucose; inositol internal standard.
Discussion
The described methods enable a rapid quantitative assessment of the composition of lignocellulosic plant biomass. The method allows the determination of the composition of such materials including the sugar composition of the matrix polysaccharides namely the hemicelluloses, the crystalline cellulose content. The throughput of the various analytical methods per person varies. Using the protocols described here, 20 samples can be processed for matrix polysaccharide compositions and 30 for crystalline cellulose content. Due to the quantitative nature of the data optimal feedstock crops, variety or genotypes can be assessed in terms of their suitability for biofuel production.
Acknowledgements
We are grateful to Matthew Robert Weatherhead for excellent technical service and John Ralph, University of Wisconsin for valuable advice, discussions, and the poplar wood sample. This work was funded by the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy (award no. DE-FG02-91ER20021) and by the US Department of Energy (DOE) Great Lakes Bioenergy Research Center (DOE BER Office of Science DE-FC02-07ER64494).
Materials
| Material Name | Typ | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Trifluoroacetic acid | Sigma-Aldrich | T6508 | ||
| myo-Inositol | Sigma-Aldrich | I5125 | ||
| Sodium Borohydride | Sigma-Aldrich | 213462 | ||
| Pyridine | J.T. Baker | 3348-01 | ||
| Acetic Anhydride | Sigma-Aldrich | 320102 | ||
| Spectromax Plus 384 | Molecular Devices | Plus384 | ||
| GC-MS | Agilent | 7890A GC/5975C MSD | ||
| 5.5mm Stainless Steel Balls | Salem Ball Company | (N/A) | ||
| 96 well plate heat spreader | Biocision | Coolsink 96F | ||
| Retsch Mill | Qiagen | TissueLyser II | ||
| Heating block | Techne | Dri-block DB-3D | ||
| Sample concentrator | Techne | FSC400D |
Referenzen
- Albersheim, P. A method for the analysis of sugars in plant cell wall polysaccharides by gas-liquid chromatography. Carbohydr. Res. 5, 340-340 (1967).
- Carroll, A., Somerville, C. Cellulosic Biofuels. Annu Rev Plant Biol. 60, 165-165 (2009).
- Foster, C. E., Martin, T., Pauly, M. Comprehensive compositional analysis of Plant Cell Walls (Lignocellulosic biomass), Part I: Lignin. Journal of Visualized Experiments. , (2010).
- Pauly, M., Keegstra, K. Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J. 54 (4), 559-559 (2008).
- Somerville, C. Toward a systems approach to understanding plant-cell walls. Science. 306 (5705), 2206-2206 (2004).
- Teeri, T. T., Brumer, H. Discovery, characterisation and applications of enzymes from the wood-forming tissues of poplar: Glycosyl transferases and xyloglucan endotransglycosylases. Biocatalysis and Biotransformation. 21, 173-173 (2003).
- UPDEGRAF, D. M. SEMIMICRO DETERMINATION OF CELLULOSE IN BIOLOGICAL MATERIALS. Anal. Biochem. 32 (3), 420-420 (1969).

