18.6:
The Nernst Equation
32,730 Views
•
•
Nonstandard Reaction Conditions
The interconnection between standard cell potentials and various thermodynamic parameters such as the standard free energy change ΔG° and equilibrium constant K has been previously explored. For example, a redox reaction involving zinc(II) and tin(II) ions at 1 M concentration with Eºcell = +0.291 V and ΔG° = −56.2 kJ is spontaneous.
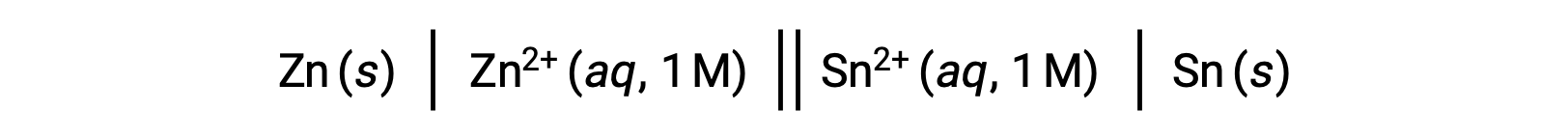
Discharge of this cell, however, results in a change in the reactant concentration and a steady decrease of the cell potential. At such a condition, however, the relationships between the cell potential and the thermodynamic parameters cannot be easily established as they only hold true at standardized conditions of concentration, temperature, and pressure (i.e., 1 M concentration, 298 K or 25 °C and a pressure of 1 atmosphere). Many redox reactions of significant scientific interest occur under nonstandard state conditions, e.g., different reactant concentrations in a galvanic cell or concentration gradients occurring across biological membranes. Hence, it becomes important to calculate the potentials of such systems.
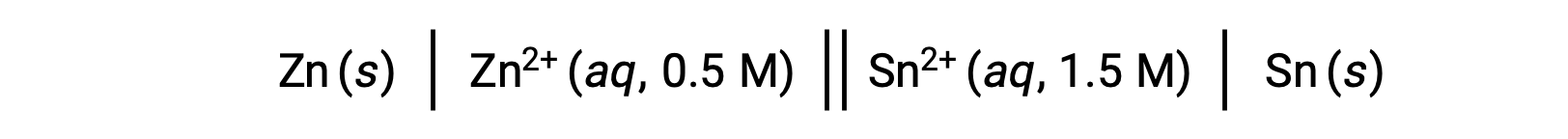
When the concentration of zinc ions in the reaction is lesser and the concentration of the tin ions greater compared to standard conditions, the spontaneity of the redox reaction can be qualitatively predicted using Le Chatelier’s Principle. Given the higher concentration of product to reactant, the reaction has a higher tendency to proceed in the direction favoring the generation of the products. This results in a higher cell potential value or Ecell than that of the E°cell value.
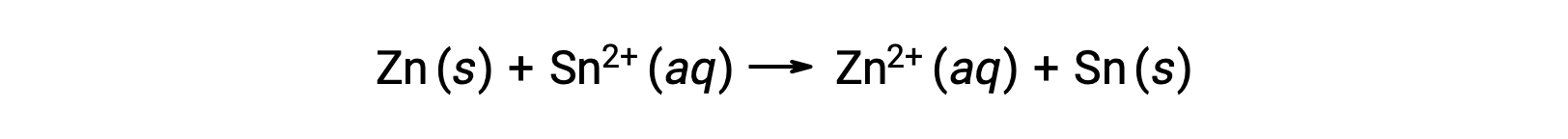
This reaction proceeds in the forward direction; however, the quantitative value of this cell potential cannot be easily determined.
Derivation of the Nernst Equation for Redox Reactions Occurring under Nonstandard Conditions
The relationship between Ecell and E°cell values can be derived from the previously established relationship between free energy changes at standard and nonstandard conditions, which is given as follows:
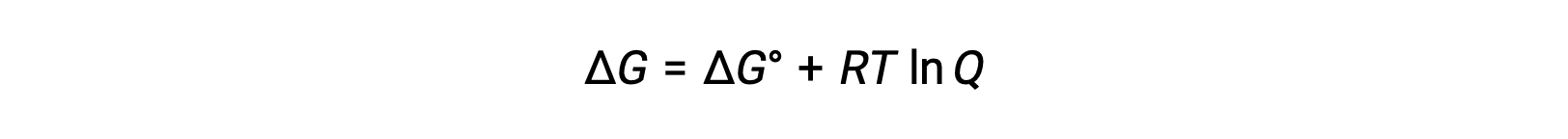
ΔG is the change in free energy, ΔG° is the standard change in free energy, R is the gas constant (value = 8.314 J/mol∙K), and Q is the reaction quotient, which accounts for the change in free energy due to the difference in the reaction mixtures’ composition. The Q value is omitted if the reactants are solid.
On substitution of the equation relating free energy change to cell potential, a modified equation is obtained, known as the Nernst equation.

The Nernst equation describes the variation in the potential of a redox system (such as a galvanic cell) from its standard state value. It is dependent on the number of electrons transferred during the redox reaction, n, the temperature measured in kelvin, T, and the reaction mixture composition given as Q.
A simplified form of the Nernst equation for most work is one in which values for the fundamental constants (R and F) and a factor converting from natural log to base-10 logarithms have been included:
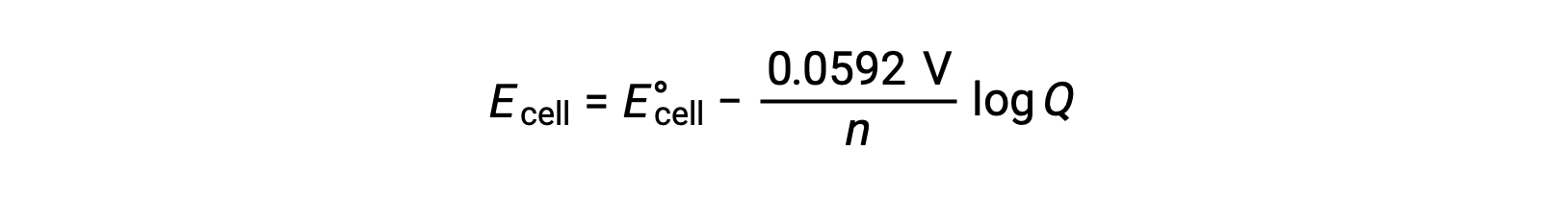
Under standard state conditions, the value of the reaction quotient Q is unity, whose logarithm is zero. This is due to the equal concentration of reactants and products at standard state conditions. Here, Ecell is equal to E°cell. A Q value less than one indicates a higher concentration of reactants, shifting the reaction equilibrium to the right and, thus, yielding a higher value of cell potential. A Q value greater than one indicates a higher product concentration, driving the reaction to the left, and a lower value of cell potential. At equilibrium, the Q value is equal to K, and the cell potential becomes zero, i.e., the reaction shows no tendency to proceed in either direction. This explains why batteries “die” on continuous discharge: the decrease in reactant concentration drives the reaction towards equilibrium and its cell potential steadily decreases to zero.
This text is adapted from Openstax, Chemistry 2e, Section 17.4: Potential, Free Energy, and Equilibrium.
