Colture pure e piastratura per striscio: isolamento di singole colonie batteriche da un campione misto
English
分享
概述
Fonte: Tilde Andersson1, Rolf Lood1
1 Dipartimento di Scienze Cliniche Lund, Divisione di Medicina delle Infezioni, Centro Biomedico, Università di Lund, 221 00 Lund, Svezia
Apparentemente impossibile da determinare, la biodiversità microbica è davvero sbalorditiva con una stima di un trilione di specie coesiste (1,2). Sebbene climi particolarmente rigidi, come l’ambiente acido dello stomaco umano (3) o i laghi subglaciali dell’Antartide (4), possano essere dominati da una specie specifica, i batteri si trovano tipicamente nelle colture miste. Poiché ogni ceppo può influenzare la crescita di un altro (5), la capacità di separare e coltivare colonie “pure” (costituite da un solo tipo) è diventata essenziale sia in ambito clinico che accademico. Le colture pure consentono ulteriori esami genetici (6) e proteomici (7), l’analisi della purezza del campione e, forse più degno di nota, l’identificazione e la caratterizzazione di agenti infettivi da campioni clinici.
I batteri hanno una vasta gamma di requisiti di crescita e ci sono numerosi tipi di mezzi nutritivi progettati per sostenere sia le specie poco esigenti che le specie esigenti (8). I mezzi di crescita possono essere preparati sia in forma liquida (come brodo) che in una forma solida tipicamente a base di agar (un agente gelificante derivato dalle alghe rosse). Mentre l’inoculazione diretta in brodo comporta il rischio di generare una popolazione batterica geneticamente diversificata o addirittura mista, la placcatura e la ri-striatura creano una coltura più pura in cui ogni cellula ha un corredo genetico molto simile. La tecnica della piastra striata si basa sulla progressiva diluizione di un campione (Figura 1), con l’obiettivo di separare le singole cellule l’una dall’altra. Qualsiasi cellula vitale (di seguito denominata unità formante colonia, CFU) sostenuta dal mezzo e dall’ambiente designato può successivamente trovare una colonia isolata di cellule figlie attraverso la fissione binaria. Nonostante i rapidi tassi di mutazione all’interno delle comunità batteriche, questo gruppo di cellule è generalmente considerato clonale. La raccolta e la ri-striatura di questa popolazione assicura di conseguenza che il lavoro successivo coinvolga solo un singolo tipo batterico.
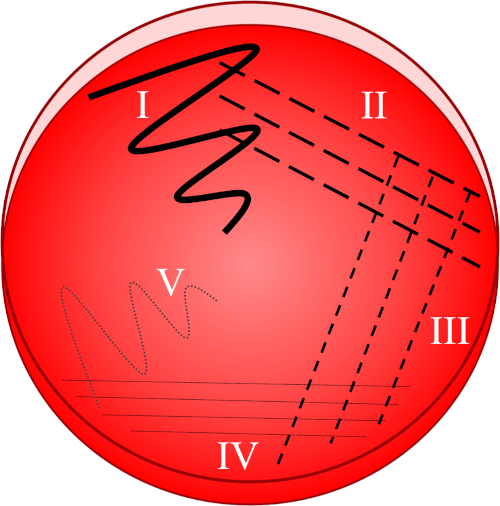
Figura 1: Una piastra striata si basa sulla diluizione progressiva del campione originale. I) L’inoculo viene inizialmente disperso utilizzando un movimento a zig-zag, creando un’area con una popolazione batterica relativamente densa. II-IV) Le strisce vengono disegnate dall’area precedente, utilizzando ogni volta un ciclo di inoculazione sterile, fino a raggiungere il quarto quadrante. V) Un movimento finale a zig-zag diretto verso il centro della placca forma una regione in cui l’inoculo è stato marcatamente diluito, permettendo alle colonie di apparire separate l’una dall’altra.
La tecnica della piastra striata può anche essere combinata con l’uso di mezzi selettivi e/o differenziali. Un mezzo selettivo inibisce la crescita di alcuni organismi(ad esempio attraverso l’aggiunta di antibiotici) mentre un mezzo differenziale aiuterà esclusivamente a distinguere l’uno dall’altro(ad esempio attraverso l’emolisi su piastre di agar nel sangue).
Alla base di tutto il lavoro in microbiologia c’è l’uso di tecniche asettiche (sterili). Ogni coltura batterica deve essere considerata potenzialmente patogena in quanto vi è il rischio di crescita involontaria di ceppi insidiosi, formazione di aerosol e contaminazione di attrezzature/personale. Per ridurre al minimo questi rischi, tutti i supporti, gli articoli in plastica, metallo e vetro vengono in genere sterilizzati attraverso l’autoclave prima e dopo l’uso, sottoponendoli a vapore saturo ad alta pressione a circa 121 ° C che elimina efficacemente le cellule persistenti. Lo spazio di lavoro viene generalmente disinfettato utilizzando etanolo sia prima che dopo l’uso. Il cappotto da laboratorio e i guanti sono sempre indossati durante il lavoro con agenti infettivi.
Procedure
Results
The initial streak-plate may contain colonies originating from cells with different genetic makeup or (depending on sample purity) from different bacterial species (Figure 2A).
Through subsequent isolation of a single colony, where all units are derived from a common mother-cell, the second streaking procedure generates a relatively clonal bacterial population, suitable for further characterization or inoculation into broth (Figure 2B).

Figure 2: A pure culture can be generated from a mixed sample through isolation of a single, secluded colony. A) Growth of a single bacterial cell (CFU) generated a clonal colony, separated from those of other species and strains. This CFU was used for subsequent streaking onto a new plate B) A second plate, where the bacterial population consists solely of cells derived from the initial CFU.
Applications and Summary
The ability to obtain and cultivate a pure bacterial colony is essential, both in clinical and academic settings. Streak plating enables the isolation of a relatively clonal cell population, originating from a shared CFU, that may be of particular interest during diagnosis or for additional characterization of the isolate. A sample is streaked onto a suitable agar-based nutrient medium and incubated until colonies become visible. An isolated colony is subsequently harvested and re-streaked onto a second plate.
References
- The Human Microbiome Project C. Structure, Function and Diversity of the Healthy Human Microbiome. Nature. 486:207-214. (2012)
- Locey KJ, Lennon JT. Scaling laws predict global microbial diversity. Proceedings of the National Academy of Sciences. 113 (21) 5970-5975 (2016)
- Skouloubris S, Thiberge JM, Labigne A, De Reuse H. The Helicobacter pylori UreI protein is not involved in urease activity but is essential for bacterial survival in vivo. Infection and Immunity. 66:4517-21. (1998)
- Mikucki JA, Auken E, Tulaczyk S, Virginia RA, Schamper C, Sørensen KI, Doran PT, Dugan H, Foley N. Deep groundwater and potential subsurface habitats beneath an Antarctic dry valley. Nature Communications. 6:6831. (2015)
- Mullineaux-Sanders C, Suez J, Elinav E, Frankel G. Sieving through gut models of colonization resistance. Nature Microbiology. 3:132-140. (2018)
- Fournier PE, Drancourt M, Raoult D. Bacterial genome sequencing and its use in infectious diseases. Lancet Infectious Diseases. 7:711-23 (2007)
- Yao Z, Li W, Lin Y, Wu Q, Yu F, Lin W, Lin X. Proteomic Analysis Reveals That Metabolic Flows Affect the Susceptibility of Aeromonas hydrophila to Antibiotics. Scientific Reports. 6:39413 (2016)
- Medina D, Walke JB, Gajewski Z, Becker MH, Swartwout MC, Belden LK. Culture Media and Individual Hosts Affect the Recovery of Culturable Bacterial Diversity from Amphibian Skin. Frontiers in Microbiology. 8:1574 (2017)
成績單
On a Petri dish, if a single bacterium undergoes multiple rounds of asexual reproduction, it will lead to the formation of a clonal colony. However, obtaining a single bacterium from a mixed sample, such as a soil suspension, can be difficult. If one loopful of this heterogeneous culture is taken, it can contain as many as one trillion individual bacteria. To spread this many bacteria out onto the surface of an agar plate and obtain a single colony, even using a zig-zag pattern, the loop would need to be dragged continuously over the surface of enough plates set side-by-side to encircle the entirety of Liberty Island. Obviously, scientists do not really use that many plates. Instead, they use a technique called streak plating.
The streak plate technique is based on progressive dilution of a bacterial sample, and it is performed over the solid media surface of a single Petri dish. To begin, the media surface is visually divided into five sections by assigning four fragments of the circumference as the first four sections, and the plate’s center as the fifth. This will effectively create five media plates out of a single Petri dish. Next, using a loopful of desired inoculum, the first section is streaked using a zig-zag pattern. Then, either a new disposable loop is used, or in the case of a wire loop, it is sterilized with a Bunsen burner, flaming it until it is red hot along the length of the wire. This use of a new loop, or flame sterilize loop, removes any remaining bacterial cells, assisting in the dilution of the bacteria. The hot loop is then cooled in the air for a few seconds before being dragged through the first section to create three to four separate lines, each carrying only a fraction of bacteria into the second section. The remaining sections are streaked in the same manner, using a sterile loop each time, and a single pass through the previous streak.
Using this cycle of streaking and sterilizing, the bacterial concentration in every subsequent section should be diluted so that the final section contains only a few discretely located bacteria. Upon incubation, these discrete bacteria multiply to produce isolated clonal colonies of daughter cells, which are referred to as Colony Forming Units, or CFUs. These can be harvested and re-streaked to ensure that subsequent work involves only a single bacterial type, referred to as a pure culture. As well as isolating single colonies from a mixed-bacterial culture, the streak plating technique is also used to select media-specific strains, determine bacterial colony morphology, or identify different bacterial species. In this video, we will demonstrate how to isolate single-bacterial colonies from a mixed-bacterial sample suspension via streak plating technique.
To begin, put on laboratory gloves and a lab coat. Next, sterilize the workspace using 70% ethanol. Next, select a suitable medium that will sustain the utilized bacterial species or strain and begin preparing the media. Here, common LB agar is prepared by weighing out ten grams of pre-formulated, powdered media and 7.5 grams of agar. Add the weighed, dried components to a glass bottle which is able to hold twice the final volume to avoid overflow. Then, add 500 milliliters of water to the bottle, and cap it semi-tightly. Sterilize the media by placing the bottle in an autoclave set to 121 degrees Celsius for twenty minutes. After completion, use heat-proof gloves or a hot pad to remove the media from the machine and then immediately twist the bottle cap to close it tightly.
For the same-day use, let the media cool down by placing the bottle into a water bath heated to approximately 45 degrees Celsius, to preserve the media in a liquid state. Alternatively, the media can be left at room temperature to store at solid state. When needed, microwave the bottle with the lid slightly open to melt the media, and allow the media to cool using a 45 degree Celsius water bath.
Next, take a sleeve of sterile Petri dishes, and with a permanent marker, label them with the investigator and media names as well as the date. Then, transfer the required volume of media into a sterile vessel, and add antibiotics or other sensitive components if necessary. Here, 50 milliliters of media is mixed with 100 microliters of Kanamycin for a final concentration of 25 micrograms per milliliter. Swirl the tube to ensure even distribution of the added components throughout the media. Slowly, so as to avoid bubble formation, pour 20 to 25 milliliters of approximately 45 degree Celsius culture medium into each of the plates. If bubbles or foam appear, swiftly remove using a regular pipette and a sterile tip. Then, immediately replace all lids to prevent contamination. Allow the agar to solidify at room temperature for at least two hours or overnight. Once solidified, store the culture plates upside down at four degrees Celsius to minimize condensation on the medium’s surface.
To streak the culture of choice, first take a clean culture plate and remove the lid. Working quickly, submerge a disposable, sterile loop into the desired inoculum and then immediately swab the loop over the first quadrant of the plate using a zig-zag motion. Replace the lid of the dish, discard the used inoculation loop, and then select a new sterile loop. Using the new loop, make three to four strokes crossing the original swab line radiating from the first quadrant, which should contain a relatively dense bacteria population into the second quadrant. Close the lid once more, and discard the loop. With a new loop, repeat this action again, but this time streaking from the second into the third quadrant. Then, with a new loop again, make another streak from the third into the fourth section of the plate. Finally, with a fresh loop, make one last stroke in a zig-zag pattern from the fourth quadrant towards the center of the plate. The bacterial prevalence will be lower in this area, ideally allowing individual colonies to be established from a single viable mother cell.
Replace the plate lid, and if appropriate for the bacterial species, seal the plate with para film to prevent airflow. Turn the culture plate upside down to prevent condensation drips, and then place at a suitable temperature for growth. Here, an incubator is set to 37 degrees Celsius. Allow the plate to incubate until bacterial colonies are visible. To generate a clonal bacterial population, select one discrete colony from this plate. Now, with the sterile loop, touch the target colony, and as before, make a streak in the first quadrant of a new plate. Continue to alternately sterilize the loop and streak the remaining quadrants of the plate as previously demonstrated, ending with the zig-zag to the center. Close the plate, and place it to incubate until discrete colonies form. Once these colonies are grown, they will typically represent pure clonal strains.
The initial streak plate may contain colonies originating from cells from different bacterial species or cells with different genetic makeup, depending upon the sample purity. Through subsequent isolation of a single colony, where all units are derived from a common mother cell, the second streaking procedure generates a relatively clonal bacterial population, suitable for further characterization or inoculation into broth.
