Synthesis of Black Seed Oil-loaded-, Alginate-based, pH-sensitive Beads Using Electrospraying Technique
Summary
A technique employing high electric voltage and a targeted, active ingredient-loaded emulsion to fabricate pH-responsive, uniform microbeads is presented.
Abstract
Black seed oil (BSO), derived from the seeds of the Nigella sativa plant, has garnered attention for its potential anti-cancer properties, particularly in the context of colon cancer. Its active compound, thymoquinone, may help inhibit cancer cell growth and induce apoptosis in colon cancer cells. Additionally, black seed oil’s anti-inflammatory and antioxidant effects could contribute to a healthier gut environment, potentially reducing cancer risk. Therefore, this study synthesized pH-sensitive alginate beads to deliver BSO into the colon in a controlled-release manner without releasing the drug at pH 1.2 (stomach), thus providing a well-defined release pattern at pH 6.8. The use of electrospray technology improves process performance by making it easier to formulate small, homogeneous beads with a higher rate of swelling and diffusion in the gastrointestinal medium.
The formulated beads were characterized by an ex-vivo mucoadhesive strength test, bead size, sphericity factor (SF), encapsulation efficiency (EE), scanning electron microscope (SEM), in vitro swelling behavior (SB), and in vitro drug release in acidic and buffer media. All these manufactured beads demonstrated modest sizes of 0.58 ± 0.01 mm and spherical shape of 0.03 ± 0.00 mm in this testing. The formulation showed promising floating and releasing properties in vitro. With a very low cumulative percentage of beads, the oil EE of 90.13% ± 0.93% was high, and the release study demonstrated more than 90% in pH 6.8 with good floating nature in the stomach. Additionally, the beads were evenly spaced throughout the intestine. The electrospraying approach used in this protocol can be reproducible, yielding consistent outcomes. Therefore, this protocol can be used for large-scale production for commercialization purposes.
Introduction
Black seed, and more especially BSO, has been used for ages to cure a wide range of illnesses due to its well-established medicinal properties. Thymoquinone is perhaps one of the most important phytochemicals found in BSO1. In recent years, researchers have studied thymoquinone's potential therapeutic benefits in vivo and in vitro, producing empirical evidence to support the use of BSO. Antihypertensive, antibacterial, antihistaminic, antifungal, analgesic, antidiabetic, lipid-lowering, and anti-inflammatory properties have all been demonstrated by these studies for BSO, which may be used to treat symptoms such as eczema, high blood pressure, asthma, cough, headache, influenza, fever, anticancer, dizziness, and activity2,3.
Applying relatively thin coverings on small droplets of liquids and dispersions, or particles of solid material, is known as microencapsulation. When it comes to oil, microencapsulated oil is usually quite valuable because some forms of oil, like BSO, are regarded as nutritious foods and offer medicinal advantages4. However, adding oils directly to the food's matrix may lead to volatilization, which can quickly cause activities to disappear as a result of exposure to oxygen and UV light5. Furthermore, the lack of control over the oils' rate of release results in an immediate and transient effect. Creating a polymeric coating around the essential oil by microencapsulation or microspherification is one method to get past these drawbacks6.
Microcapsules, also known as microspheres, shield the oils from harmful environmental conditions7. This process has been widely used to increase drug efficacy, preserve drug contents, enable time-released tablets, improve taste masking, reduce flavor loss during product shelf life, prolong mouthfeel, and separate incompatible ingredients in a single dosage8. Microencapsulation also aids in maintaining metabolic absorption, controlling the rate of oil release, and maintaining the appropriate concentrations to yield the intended result at a particular location9.
Electro-hydrodynamic encapsulation is a straightforward and adaptable method. The active substance is housed in the inner core of a microcapsule, which is composed of an exterior shell. In this regard, it features a fairly strong matrix to guarantee that the active component can be disseminated more effectively rather than a clearly defined nucleus. Prior to sphericyclation, the active substance and polymer solution must be combined to produce the microspheres9. On the other hand, because oil is volatile, microencapsulating it can be extremely difficult and requires careful temperature control.
There are various methods for encapsulating oils. For example, certain oils need to be encapsulated at low temperatures to prevent the breakdown or volatilization of their bioactive components. To create micro- and nano-sized structures, electrohydrodynamic atomization (EHDA) has been extensively studied by researchers10. In this sense, the processing conditions, which include flow rate, applied voltage, and nozzle size, as well as the collection distance properties of the polymeric solution, are the two primary factors that must be taken into account to produce the desired particle size or morphology11,12.
In this investigation, alginates — a type of naturally occurring polysaccharides fit for oral ingestion — were used to encapsulate the BSO. Brown seaweeds contain alginate, an anionic polymer that occurs naturally. It is made up of two monomeric structures: α-L-guluronic (G) and 1-4βD-mannuronic (M) acid13. Its polymer is non-toxic14, has a high degree of biocompatibility, is inexpensive, and degrades effectively15. It is, therefore, frequently employed in the biotechnology and engineering sectors.
Alginates are the material of choice for encapsulation by ionic gelation because they may create a cross-linked structure between the G groups of various alginate chains by forming ionic connections with divalent cations like Sr2+, Ca2+, or Zn2+ ions. The gelation process can be adequately characterized by the egg-box model, which limits the divalent cation to two carboxyl groups on the side-by-side alginate molecules. It has been suggested that the hydrogel characteristics of sodium alginate beads may regulate the release of macromolecules and small molecules. The sodium alginate beads may cling to the intestinal mucosa for an extended length of time due to their muco-adhesive qualities. Moreover, alginate offers a shield that may shield oils from external elements such as acidic media16 and transfers oils into the delivery channels of the gastrointestinal tract17. It has since been employed in research to aid in the site-specific administration of medication to mucosal tissues18,19.
The electro-hydrodynamic approach was used in this study to investigate the viability of emulsifying commercial oils to create capsules20. Here, the electro-hydrodynamic approach was used to generate and analyze alginate-BSO-loaded microspheres20. This study evaluated a number of other factors, including the microspheres' SF, ex-vivo, muco-adhesive properties, EE%, physical appearance, size distribution, and zeta potential; attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy was utilized to test for chemical compatibility20.
Protocol
1. Preparation of alginate-BSO emulsion
- Disperse 10% w/v BSO in 1% w/v sodium alginate solution containing 1, 3, and 5% w/v lecithin in a 50 mL beaker.
- Obtain a nano-emulsion by using an ultrasonic homogenizer. Set the power level to 20%. Run the homogenizer for 55 s by clicking the start button to complete the process.
2. Characterization of alginate-BSO emulsion
- Analyze the zeta potential and particle size distribution
- Take 0.1 mL of the freshly prepared emulsion in a 25 mL glass beaker and dilute it with 9.9 mL of distilled water.
- Take 2.5 mL of this diluted solution into a 3 mL quartz-cuvette and place the cuvette in a measuring chamber.
- Open the cover and place the cuvette inside the device, making sure the cuvette is oriented correctly with respect to the path of the light beam. Click the measurement icon.
- Take out the cuvette. Retrieve the sample or properly dispose of it.
- Save the data as a pdf file in a personal folder for further use.
- Determine emulsion stability (ES)
- Take 5 mL of freshly prepared emulsion in 10 mL centrifuge tubes. Centrifuge the emulsions (n = 3) for 5 min at 894 × g.
- Using equation (1), determine the ES based on the phase separation interface position.
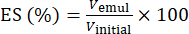 (1)
(1)
Where Vemul is the volume of remaining emulsion after centrifugation and Vinitial is the volume of initial emulsion.
- Bead preparation
- BSO alginate beads
- Prepare BSO alginate beads using the electrospray technique called EHDA. Employ a weight by volume (w/v) BSO emulsion that is composed of 10% BSO, 1% sodium alginate, and 3% lecithin solution).
- Using a syringe pump to regulate the flow rate, load the emulsion into a 10 mL plastic syringe and push it through a 22 G needle. Attach the needle tip to the positive electrode of a high voltage power supply.
- As the collector, use a grounded beaker with 50 mL of 1% w/w calcium chloride (gelling bath) in it. Alternate the dripping flow rate between 1 mL/min and 3 mL/min at voltages of 3, 5, and 7 kV while maintaining a distance of 10 cm above the calcium chloride solution's surface.
- To confirm full gelation, leave the beads in the gelling bath for 30 min while agitating them. Use a stainless-steel filter to remove the beads from the gelling bath and wash the gathered beads with ultrapure distilled water.
- Let the beads dry for 16 h at room temperature on a lab bench. Use Equation (2) to calculate the percentage yield of beads.
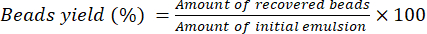 (2)
(2)
- BSO-free alginate beads
- Prepare a 1% w/v solution of sodium alginate. Add lecithin at concentrations of 1%, 3%, and 5% w/v to the solution. Mix the solution thoroughly until the lecithin is dissolved completely.
- Use the solution from step 2.3.2.1 to prepare BSO-free alginate beads as described in steps 2.3.1.2-2.3.1.5. Calculate the yield using equation (2).
- BSO alginate beads
3. Bead characterization
- Determination of size and shape
- To ascertain the size and shape of the beads, use an image analyzer. Take photos using a digital camera of the wet and dried beads.
- Then, measure bead diameter using the instrument's preinstalled scale bar. Using the diameter values, calculate the SF from the obtained diameter values using equation (3):
SF = (3)
(3)
Where Dmax represents the largest diameter that goes through the center of a bead (in mm), while Dper refers to the diameter that is perpendicular to Dmax and passes through the bead's center (in mm).
NOTE: An SF of zero denotes an ideally spherical bead, with increasing SF values signifying greater deviation from a spherical shape. Furthermore, beads are considered spherical if their SF is 0.05 or lower.
4. Determination of EE%
- Disintegrate the beads in phosphate-buffered saline (PBS) to return them into emulsion. Measure the absorbance of the resulting emulsion at 600 nm using a UV-vis spectrophotometer.
- Use the absorbance value to represent the turbidity of the emulsion. Create a standard curve using a known amount of BSO in the emulsion.
- Calculate the EE% using equation (4):
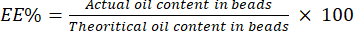 (4)
(4)
5. Scanning electron microscopy (SEM)
NOTE: Use SEM to observe the microstructure and surface morphology of the BSO alginate beads.
- To inspect the inside of the dried beads, cut a few of them. Spot the cut beads onto aluminum stubs and paste them using carbon adhesive tapes.
- Sputter-coat the beads with a carbon sputter module in a vacuum evaporator in an argon atmosphere. Apply a thickness of 100 Å and 50 Å for the carbon coating.
- Acquire images of the coated beads at high vacuum with a voltage accelerator between 10 kV and 15 kV.
6. Determine drug-excipient interaction using ATR-FTIR
- Set the instrument wave numbers between 4,000 cm−1 and 400 cm−1 using ambient air as a background and a resolution of 1 cm−1. See Supplemental File 1.
- Record spectra of BSO, sodium alginate, lecithin, calcium chloride, BSO-free beads, alginate-BSO beads, and physical mixture of active ingredient and excipients (sodium alginate, lecithin, calcium chloride, and BSO) separately.
- Place the sample (5-10 mg) on the sampling area. Adjust 20 scans, resolution 4, gauge force 80, pressure arm with a flat tip. Verify that the Auto Increment remains set to the Blank option so that the spectrum is automatically stored in the desired folder.
- To begin the sample measurement, click the [Sample] button. After selecting [Sample], since there is no waiting period, be sure to have the sample ready and the pressure clamp reduced. Analyze all the samples individually. Individually analyze all the recorded spectra using spectroscopy software.
7. Differential scanning calorimetry (DSC)
NOTE: The thermal properties and compatibility of the BSO-loaded beads were investigated using DSC (Supplemental File 1).
- Seal beads weighing ~3.20 mg in a regular aluminum pan. Heat the samples at a rate of 10 °C/min while being analyzed at a temperature range of 50-350 °C under a stream of nitrogen flowing at a rate of 20 L/min.
8. Swelling characteristics of beads
- Prepare 100 mg of dried alginate-BSO beads.
- Prepare simulated intestinal fluid (SIF) and simulated gastric fluid (SGF) in a clean, dry mixing vessel of a suitable size-6 L, 10 L or 25 L. Add purified water to approximately 33% of the required volume-2 L, 3 L or 8 L, and transfer the contents of the bottle of concentrate into the vessel. Rinse the bottle with purified water and add the rinses and purified water to the mixing vessel to obtain the required volume; mix thoroughly. Measure the pH and proceed if within specification; adjust the pH if necessary.
- Submerge the beads in 50 mL of media containing simulated intestinal fluid (SIF) and simulated gastric fluid (SGF). Maintain the conditions for 2 h at 37 ± 0.5 °C.
- Remove the inflated beads and filter them through a metal mesh at predefined intervals of 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, and 120 min or more. Use a paper towel to remove extra fluid from the swollen beads.
- Measure the weight of wiped beads using an electronic analytical balance. Determine the percentage of swelling index (%SI) using equation (5):
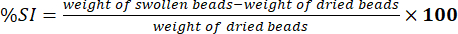 (5)
(5)
Representative Results
Preparation of BSO-loaded alginate microbeads
Figure 1 represents the experimental setup to prepare BSO-loaded alginate microbeads. The amount of lecithin utilized had a considerable impact on the stability of the BSO emulsion. Emulsions made with all three lecithin concentrations were comparatively stable. The centrifugation method (894 × g, 5 min) was used in this experiment to assess the stability of the emulsion20. The results verified that surfactant, not centrifugation, had a greater impact on the stability of BSO emulsion. The zeta potential and particle size distribution of the emulsion were characterized as shown in Table 1 and Figure 2.
Characterization of beads
Size, shape, and EE
The formulated microbeads' size, shape, and EE are summarized in Table 2. The dried beads ranged from 1.86 ± 0.02 mm (F2 and F3) to 0.58 ± 0.01 mm (F8), and the wet beads were between 4.76 ± 0.01 mm (F3) and 1.50 ± 0.02 mm (F8). The electrical voltage and emulsion flow rate were two of the main parameters that affected the beads' size and sphericity. Both the SF and the size of the beads increased with increased flow rate, while these variables decreased with increased electrical voltage. Nevertheless, the SF and bead size did not drop at a certain point when the electrical voltage and flow rate were both increased proportionately. This study finds that beads produced at a flow rate of 2 mL/min and an electrical voltage of 7 kV are consistently the same size, shape, and weight. The researchers measured the bead size and found that it was 0.58 ± 0.01 mm. For both wet and dry bead formulations, the EE ranged from 67.20 ± 1.46% (F1) to 104.50 ± 4.04% (F8) and from 59.92 ± 0.90% (F1) to 90.13 ± 0.93% (F8), in that order. The proportion of EE rose together with the voltage and flow rate, which might be explained by the high voltage, which increased the cross-linking's binding to the CaCl2 gelling solution and produced additional spaces for ionic cross-linking. As a result, more oil was trapped and oil leakage from the polymer matrix was avoided.
Bead weight uniformity
The weight variation coefficients of alginate beads loaded with BSO ranged from 1.37% to 3.93%. According to the latest research, the average weight of all the BSO-loaded beads did not differ by more than 5%.
ATR-FTIR
To determine the interaction within ingredients, ATR-FTIR was performed (Figure 3). Sodium alginate exhibited characteristic spectral bands at 3400 cm−1 for O-H stretching and at 1595 and 1408 cm−1 for C=O and C-C groups as a result of stretching vibration. Two distinct bands were also found at 1600 and 1405 cm−1 in a prior investigation, which were linked to the symmetric and asymmetric stretching vibrations of the -COOH groups, respectively. In addition, the C-H group was responsible for the bands seen in the sodium alginate spectra at 1316 and 1025 cm−1. In contrast, the lecithin spectra revealed three bands at 2922, 2853, and 1465 cm−1, corresponding to the C=O, C-H, and C-C groups. The BSO peaks that stood out the most were found at 2953 and 2853 cm−1 (C-H in CH2 and C=C-H, correspondingly). Moreover, because carbon chains are common in fatty acids, the bands that emerged at 1,753, 1,653, and 1,453 cm−1 suggested C=O, C=C, and C-H, respectively.
DSC
DSC analysis was used to determine the temperature behavior of alginate beads loaded with BSO and their interactions with other extractants during the preparation and development process. Polyelectrolyte breakdown is caused by depolymerization and dehydration processes, most likely polyelectrolyte oxidation reactions and partial decarboxylation of the protonated carboxylic groups (Figure 4).
SEM
Using SEM, images of the surface of the dried beads optimized with BSO loading were captured on camera. This demonstrated that the beads had a smooth surface, were spherical in shape, and had numerous pores and channels across their outer layer (Figure 5).
Bead swelling behavior
Bead swelling is a significant behavior that affects the drug release pattern of polymeric materials21. To evaluate the SB, beads containing BSO were tested in SGF (0.1 N HCl, pH 1.2) and SIF (pH 6.8) (Figure 6).
Alginate shrinking in an acidic pH may account for the first decreased swelling index of bead formulations F1 to F9 in SGF compared to SIF, which indicates a pH-sensitive SB. Consequently, the beads displayed a higher percentage of SI in SIF after 30-90 min. The SB of the beads in an alkaline pH can be explained by the exchange of calcium ions, whereby the presence of calcium-sequestrant phosphate ions binds the carboxylic groups of the cross-linked alginate lecithin matrix to the sodium ions in phosphate buffer. In addition, the inflated cross-linked BSO-loaded alginate beads disintegrated and eroded in 100-110 min, and at 120 min, all formulations had completely eroded.
This study's primary goal is to synthesize Black Seed Oil-loaded, Alginate-based, pH-sensitive Beads Using Electrospraying Technique to release the drug where it is normally absorbed — in the intestine. In an acidic pH of 1.2, the beads shrank or retained their original shape, which lessened their propensity to disintegrate. As opposed to this, BSO-loaded alginate beads quickly swelled when exposed to SIF (pH 6.8). After 120 min, all nine formulations (F1-F9) had entirely dissolved in the SIF. This resulted from the presence of phosphate ions in the medium, which increased the solubility of the sodium alginate complex at higher pH levels. The decoupling of alginate-sodium and the gel's subsequent breakdown in the phosphate buffer were caused by the phosphate ions' higher affinity for Ca2+ than for alginate ions.
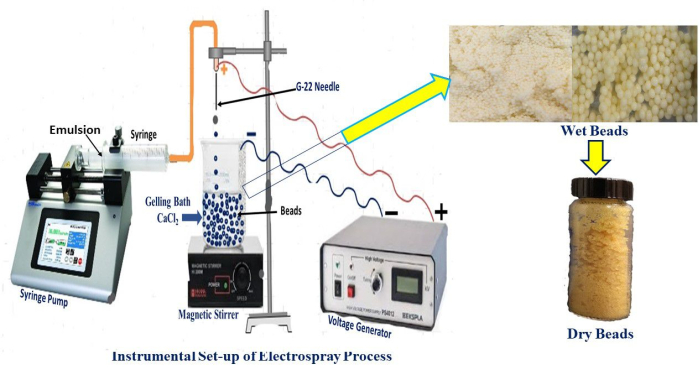
Figure 1: Experimental setup of the electrohydrodynamic atomization technique for the fabrication of alginate microbeads. Real-time setup for beads production and freshly prepared wet beads. Please click here to view a larger version of this figure.
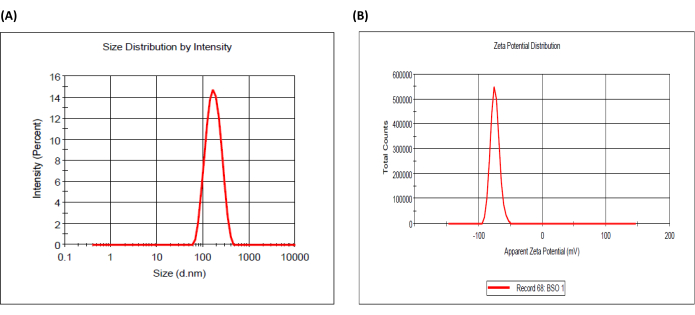
Figure 2: Particle size distribution and zeta potential of black seed oil-loaded emulsion. (A) Size distribution and (B) zeta potential distribution. Please click here to view a larger version of this figure.
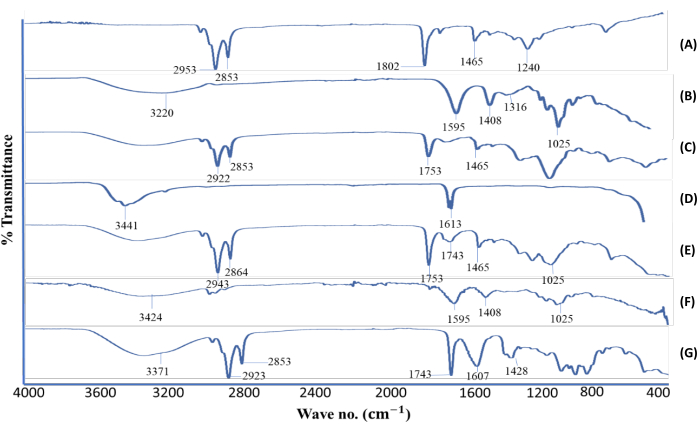
Figure 3: Attenuated total reflectance-Fourier transform infrared spectra. (A) BSO, (B) sodium alginate, (C) lecithin, (D) calcium chloride, (E) BSO-free beads, (F) alginate-BSO beads, and (G) physical mixture of active ingredient and excipients (sodium alginate, lecithin, calcium chloride, and BSO). Abbreviation: BSO = black seed oil. This figure was modified from Azad et al.20. Please click here to view a larger version of this figure.
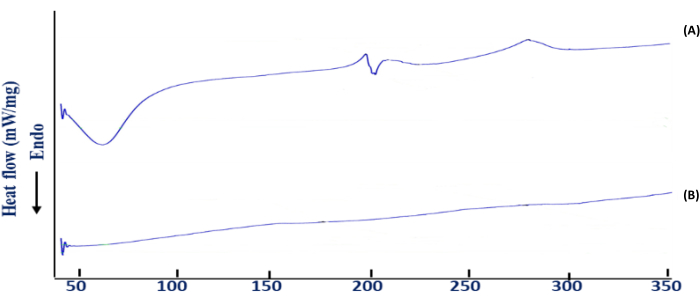
Figure 4: Differential scanning calorimetry (DSC) thermogram. (A) BSO-free beads and (B) BSO-loaded beads. This figure was modified from Azad et al.20. Please click here to view a larger version of this figure.
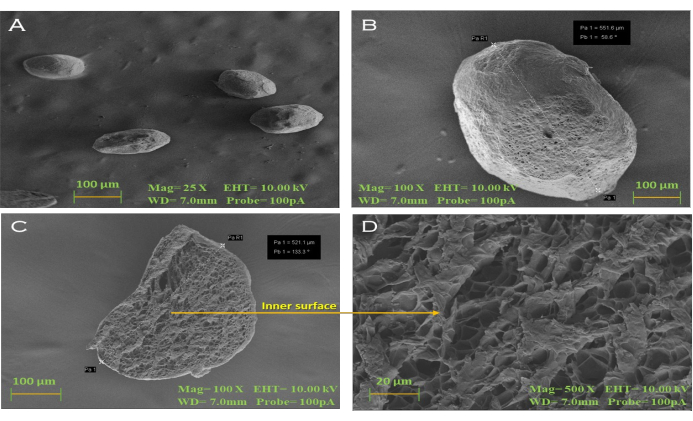
Figure 5: Scanning electron microphotographs. (A, B) Alginate-BSO dried beads showing smooth surface morphology (25x and 100x); the presence of pores and channels seen in the dried bead slice with a magnification of (C) 100x and (D) 500x. Scale bars = 100 µm (A-C), 20 µm (D). This figure was modified from Azad et al.20. Please click here to view a larger version of this figure.
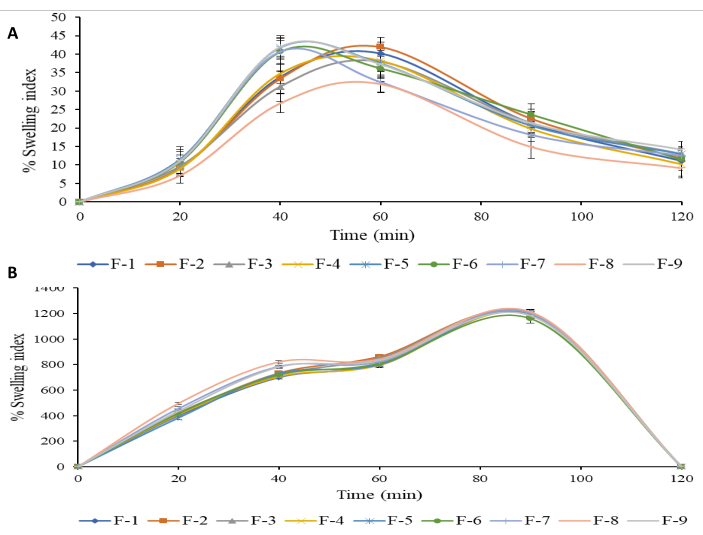
Figure 6: Swelling behavior of optimized black seed oil-loaded alginate beads. (A) Acidic media (pH 1.2) and (B) buffer media (pH 6.8) [mean ± SD, n = 3). Please click here to view a larger version of this figure.
| % lecithin conc. | Particle size (nm) | PDI | Zeta potential (mV) | ES (%) | |||
| Before | After centrifugation | Before | After centrifugation | Before | After centrifugation | ||
| 1 | 253.32±4.65 | 282.93±14.11 | 0.392±.03 | 0.333±.06 | -43.1±.36 | -37.6±2.2 | 92.50±0.23 |
| 3 | 334.51±12.82 | 347.44±15.14 | 0.315±.04 | 0.358±.12 | -46.9±2.26 | -43.5±1.32 | 94.83±0.42 |
| 5 | 430.13±14.23 | 463.23±8.91 | 0.422±.04 | 0.548±.11 | -51.4±2.75 | -45±1.01 | 96.50±0.40 |
Table 1: Particle size distribution and zeta potential analysis of black seed oil-loaded emulsion. *(n = 3, mean ± SD). Abbreviations: PDI = polydispersity index; ES = emulsion stability.
| Code | Voltage (kV) | Flow rate (ml/min) | Bead sizea (mm) | Sphericity Factora (mm) | Encapsulation Efficiencya (%) | |||
| Wet | Dry | Wet | Dry | Wet | Dry | |||
| F1 | 3 | 1 | 4.47±0.01 | 1.82±0.01 | 0.02±0.00 | 0.04±0.00 | 67.20±1.46 | 59.92±0.90 |
| F2 | 3 | 2 | 4.52±0.03 | 1.86±0.02 | 0.01±0.00 | 0.03±0.00 | 68.22±3.09 | 65.89±1.57 |
| F3 | 3 | 3 | 4.76±0.01 | 1.86±0.01 | 0.02±0.01 | 0.04±0.00 | 70.76±0.86 | 66.34±1.89 |
| F4 | 5 | 1 | 3.12±0.01 | 0.99±0.04 | 0.02±0.01 | 0.01±0.01 | 70.11±0.51 | 70.11±0.51 |
| F5 | 5 | 2 | 2.83±0.05 | 0.80±0.02 | 0.02±0.01 | 0.02±0.02 | 86.89±1.67 | 86.89±1.67 |
| F6 | 5 | 3 | 3.30±0.01 | 0.90±0.02 | 0.03±0.01 | 0.01±0.01 | 86.63±0.83 | 86.12±0.15 |
| F7 | 7 | 1 | 1.86±0.02 | 0.72±0.01 | 0.01±0.00 | 0.04±0.00 | 82.16±2.50 | 78.67±0.90 |
| F8 | 7 | 2 | 1.50±0.02 | 0.58±0.01 | 0.02±0.00 | 0.03±0.00 | 104.50±4.04 | 90.13±0.93 |
| F9 | 7 | 3 | 2.58±0.05 | 0.93±0.01 | 0.02±0.00 | 0.03±0.01 | 96.04±3.61 | 89.82±154 |
Table 2: Effect of voltage (kV) and flow rate (mL/min) on bead size, sphericity factor, and encapsulation efficiency. n = 3, mean ± S.D. This table was modified from Azad et al.20.
Supplemental File 1: Measurement of particle size and distribution, ATR-FTIR, and DSC. Please click here to download this File.
Discussion
Using the EHDA process, BSO-loaded alginate microbeads were created as a pH-sensitive carrier. The beads' network exhibited pH-dependent swelling and drug-release behavior due to the abundant presence of carboxylic acid groups. The strong intermolecular hydrogen bonding between the polymer chains was revealed to be the reason behind the decreased swelling character of BSO-loaded beads at pH 1.2. BSO-loaded beads may benefit from this reduced SB for pH-sensitive medication administration. pH had an impact on the BSO released from the beads containing BSO. These findings suggest that BSO-loaded beads may provide a secure oral medication delivery method for better, targeted drug administration to the gut.
The particle size of the emulsion increased with an increase in lecithin concentration. This phenomenon may be attributed to the rise in viscosity associated with higher lecithin levels, which could affect the size reduction capabilities of the ultrasonic homogenizer. Furthermore, increased lecithin concentrations contribute to a denser surfactant layer around the oil droplets, resulting in larger particle sizes. The emulsion's microscopic appearance suggests that the emulsions maintained their stability well. The concentration of lecithin can affect the distribution of particle size and zeta potential20. The emulsions showed a non-homogeneous particle size distribution, which may be due to the manual sonication procedure. Zeta potential has a key role in maintaining the stability of colloidal systems21. The droplets within the emulsion exhibited a significantly negative charge, which can be attributed to the alginate's negative charge arising from the hydroxyl and carboxyl functional groups within its molecular structure22.
In the characterization of the microbeads, size, shape, and EE are significant parameters influenced by the processing conditions. The relationship between the electrical voltage, emulsion flow rate, and the resulting microbead characteristics indicates a complex interplay that determines the final properties of the beads. When increasing the flow rate, the bead size and SF also increase. On the other hand, increasing the electrical voltage decreases the bead size and SF but increases EE. However, if the electrical voltage and flow rate are increased proportionally, the bead size and SF do not decrease at a specific point20. The capacity for cross-linking at the alginate beads' exterior also plays an important role in enhancing the EE for oils within alginate. A previous study showed an optimal EE of 98.30% for linseed oil encapsulated in alginate beads that were oven-dried, with bead sizes ranging between 1.90 mm and 2.94 mm and an SF between 0.044 and 0.168. Despite these high encapsulation efficiencies, the release of the drug to the intended site in the digestive tract was found to be only 83%23. Another investigation yielded a similar EE of 98.7%, with bead sizes at 2.13 mm and an SF of 0.08 mm. Conversely, the dry, electrosprayed alginate beads loaded with BSO in this study demonstrated an EE of 90.13 ± 0.93%, with over 90% of the substance being successfully released at the intended site in the SIF, according to our release study. This suggests that our alginate beads were effectively able to encapsulate and nearly completely release BSO at the desired location24.
According to the standards set by the USP Pharmacopeia, all formulations (F1-F9) satisfied the requirements for weight uniformity25. This uniformity in weight reflects consistent encapsulation of BSO within the alginate beads, facilitated by the use of electrohydrodynamic techniques. The optimal bead size and shape, suitable for oral administration, were achieved under specific conditions, which reveals the importance of precisely controlled manufacturing processes. Furthermore, the EE, which varied with voltage and flow rate, suggests that the process parameters can be fine-tuned to maximize BSO encapsulation. This has significant implications for the bioavailability and effectiveness of the encapsulated compound, with higher encapsulation efficiencies potentially leading to better therapeutic outcomes.
The uniformity in bead weight, along with the ATR-FTIR and SEM analyses, provides insight into the consistency and chemical integrity of the alginate beads26,27. The ATR-FTIR analysis confirmed the presence of key functional groups and the successful incorporation of BSO within the alginate matrix28, while SEM images illustrated the spherical shape and smooth surface of the beads, essential for consistent release behavior. The SB study illustrated the pH-sensitive swelling properties of the beads, which is significant for targeted drug release in the intestine, ensuring that BSO is released where it can be most effectively absorbed29. This pH-responsive behavior exemplifies the potential of alginate-based microbeads as a promising delivery system for BSO, enabling targeted, controlled release within the gastrointestinal tract.
As a result of the study's successful development of pH-sensitive alginate beads for the regulated release of black seed oil, thymoquinone delivery for the treatment of colon cancer is highlighted. The manufacturing of uniformly sized beads with desired swelling properties and good encapsulation efficiency was made easier by the application of electrospray technology. These beads showed good floating properties, as seen by a release rate of more than 90% in a pH 6.8 environment, guaranteeing targeted delivery inside the gastrointestinal tract. The findings point to a possible strategy for increasing black seed oil's bioavailability while reducing its early release in the stomach. This technique has a lot of potential for large-scale manufacturing, opening the door for potential commercialization of cancer treatments in the future.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R30), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. This research was funded by the Researchers Supporting Project number (RSPD2024R811), King Saud University, Riyadh, Saudi Arabia.
Materials
| 10 mL Centrifuge Tubes | Globe Scientific | 22-171-624 | |
| 22 G needle | Sigma-Aldrich (St.Louis, Missouri, USA). | CAD4172 | |
| 3 mL quartz-cuvette | Sigma-Aldrich (St.Louis, Missouri, USA). | Z276669 | |
| 50 mL beaker | |||
| Aluminum stubs | |||
| An electronic analytical balance | |||
| ATR-FTIR | Bruker Malaysia Sdn Bhd, Kawasan Perindustrian Temasya, 40150 Shah Alam, Selangor, Malaysia. | ||
| Black seed oil | IKOP Pharmaceutical Ltd. (IKOP, Faculty of Pharmacy, IIUM, 25200 Kuantan, Pahang, Malaysia | B182111 | Active ingredient |
| Calcium chloride dehydrate, CaCl2 · 2H2O | Sigma-Aldrich (St.Louis, Missouri, USA). | 21074 | Gelling agent |
| Carbon adhesive tapes | |||
| Centrifuge | |||
| Differential scanning calorimetry | |||
| Digital camera | |||
| Grounded beaker | |||
| High guluronic acid content Sodium alginate (mw. 97,000) with medium viscosity (40 – 100 mPa s) | Sigma-Aldrich (St.Louis, Missouri, USA). | W201502 | Polymer |
| High voltage power supply | |||
| Isopropyl alcohol | Sigma-Aldrich (St.Louis, Missouri, USA). | W292912 | ATR-FTIR cleaning purpose |
| Lecithin | Sigma-Aldrich (St.Louis, Missouri, USA). | P7568 | Surfactant |
| Microscope | |||
| Paper towel | |||
| Scanning electron microscopy | |||
| Simulated gastric fluid | Sigma-Aldrich (St.Louis, Missouri, USA). | 1651 | Release media and swelling media |
| Simulated intestinal fluid | Sigma-Aldrich (St.Louis, Missouri, USA). | 84082-64-4 | Release media and swelling media |
| Spectroscopy software | |||
| Stainless-steel filter | |||
| Syringe pump | SEN JIN SDN BHD Malaysia, Taman Desaria, 46150 Petaling Jaya, Selangor Darul Ehsan Malaysia | ||
| Ultrapure distilled water | Supplied by institutional lab | ||
| Ultrasonic homogenizer | SEN JIN SDN BHD Malaysia, Taman Desaria, 46150 Petaling Jaya, Selangor Darul Ehsan Malaysia | ||
| UV-vis spectrophotometer. | |||
| Vacuum evaporator | SEN JIN SDN BHD Malaysia, Taman Desaria, 46150 Petaling Jaya, Selangor Darul Ehsan Malaysia | ||
| Voltage accelerator | SEN JIN SDN BHD Malaysia, Taman Desaria, 46150 Petaling Jaya, Selangor Darul Ehsan Malaysia | ||
| Zetasizer Nano-ZS | (Malvern Zetasizer Nano series Nano-S and Nano-Z, Malvern Instruments Ltd., Worcestershire, UK) |
References
- Benavides, S., Cortés, P., Parada, J., Franco, W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem. 204 (8), 77-83 (2016).
- Agbaria, R., Gabarin, A., Dahan, A., Ben-Shabat, S. Anticancer activity of Nigella sativa (black seed) and its relationship with the thermal processing and quinone composition of the seed. Drug Des Devel Ther. 9 (1), 3119 (2015).
- Wang, D., Qiao, J., Zhao, X., Chen, T., Guan, D. Thymoquinone inhibits IL-1β-induced inflammation in human osteoarthritis chondrocytes by suppressing NF-κB and MAPKs signaling pathway. Inflammation. 38 (7), 2235-2241 (2015).
- Beyki, M. et al. Encapsulation of Mentha piperita essential oils in chitosan-cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind Crop Prod. 54 (3), 310-319 (2014).
- Hosseini, S. M. et al. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. Inter J Biol Macromol. 62 (11), 582-588 (2013).
- Banerjee, S. et al. Influence of process variables on essential oil microcapsule properties by carbohydrate polymer-protein blends. Carbohydr Polym. 93 (2), 691-697 (2013).
- Sebe, I., Szabó, E., Zelkó, R. Advances in drug delivery via electrospun and electrosprayed formulations. In Emerging Drug Delivery and Biomedical Engineering Technologies. CRC Press, 71-104 (2023).
- Akram, N. et al. Fabrication and characterization of PVA-WPI based nanofiber mats for improved viability of Lactobacillus rhamnosus GG. Foods. 12 (21), 3904 (2023).
- Azad, A. K., Sinan M. A. A., John F. K., Bappaditya C, Hriday B. Electro-hydrodynamic assisted synthesis of lecithin-stabilized peppermint oil-loaded alginate microbeads for intestinal drug delivery. Int J Biol Macromol. 185 (8), 861-875 (2021).
- Chan, E. S. Preparation of Ca-alginate beads containing high oil content: Influence of process variables on encapsulation efficiency and bead properties. Carbohydr Polym. 84 (4), 1267-1275 (2011).
- Xie, J., Jiang, J., Davoodi, P., Srinivasan, M. P., Wang, C. H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem Eng Sci. 125 (3), 32-57 (2015).
- Zamani, M., Prabhakaran, M. P., Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int J Nanomed. 8 (8), 2997 (2013).
- Husain, O., Lau, W., Edirisinghe, M., Parhizkar, M. Investigating the particle to fibre transition threshold during electrohydrodynamic atomization of a polymer solution. Mater Sci Eng. C. 65 (8), 240-250 (2016).
- Wan, L. Q. Calcium concentration effects on the mechanical and biochemical properties of chondrocyte-alginate constructs. Cell Mol Bioeng. 1 (3), 93-102 (2008).
- Baimark, Y., Srisuwan, Y. Preparation of alginate microspheres by water-in-oil emulsion method for drug delivery: Effect of Ca2+ post-cross-linking. Adv Powder Technol. 25 (5), 1541-1546 (2014).
- Paques, J. P., Sagis, L. M. C., van Rijn, C. J. M., van der Linden, E. Nanospheres of alginate prepared through w/o emulsification and internal gelation with nanoparticles of CaCO3. Food Hydrocoll. 40 (10), 182-188 (2014).
- Suksamran, T. Biodegradable alginate microparticles developed by electrohydrodynamic spraying techniques for oral delivery of protein. J Microencapsul. 26 (7), 563-570 (2009).
- Wang, H., et al. Characterization, release, and antioxidant activity of curcumin-loaded sodium alginate/ZnO hydrogel beads. Int J Biol Macromol. 121 (1), 1118-1125 (2019).
- Bera, H., Boddupalli, S., Nayak, A. K. Mucoadhesive-floating zinc-pectinate-sterculia gum interpenetrating polymer network beads encapsulating ziprasidone HCl. Carbohydr Polym. 131 (10), 108-118 (2015).
- Azad, A. K., et al. Encapsulation of black seed oil in alginate beads as a pH-sensitive carrier for intestine-targeted drug delivery: In vitro, in vivo and ex vivo study. Pharmaceutics. 12 (3), 219 (2020).
- Azad, A. K., et al. A dataset of microstructure features of electro-hydrodynamic assisted 5-fluorouracil-grafted alginate microbeads and physicochemical properties for effective colon targeted carriers drug delivery. Data in Brief. 53 (4), 110202 (2024).
- Danaei, M., et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 10 (2), 57 (2018).
- Piornos, J. A., Burgos-Díaz, C., Morales, E., Rubilar, M., Acevedo, F., Highly efficient encapsulation of linseed oil into alginate/lupin protein beads: Optimization of the emulsion formulation. Food Hydrocoll. 63 (2), 139-148 (2017).
- Morales, E. Alginate/Shellac beads developed by external gelation as a highly efficient model system for oil encapsulation with intestinal delivery. Food Hydrocoll. 70 (9), 321-328 (2017).
- Nikoo, A. M., Kadkhodaee, R., Ghorani, B., Razzaq, H., Tucker. N. Electrospray-assisted encapsulation of caffeine in alginate microhydrogels. Int J Biol Macromol. 116 (9), 208-216 (2018).
- Shao, L. et al. Effect of cold-spray parameters on surface roughness, thickness and adhesion of copper-based composite coating on aluminum alloy 6061 T6 substrate. Processes. 11 (3), 959 (2023).
- Li, W. et al. Effects of spraying parameters and heat treatment temperature on microstructure and properties of single-pass and single-layer cold-sprayed Cu coatings on Al alloy substrate. Surf Coat Technol. 30 (490), 131184 (2024).
- US Pharmacopoeia National Formulary, USP 23/NF 18. United States Pharmacopoeia Convention Inc.: Rockville, MD, USA (2000).
- Chen, Y.-C., Ho, H.-O., Liu, D.-Z., Siow, W.-S., Sheu, M.-T. Swelling/floating capability and drug release characterizations of gastroretentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose. PLoS One. 10 (1), e0116914 (2015).

.
