Embryo Microinjection Techniques for Efficient Site-Specific Mutagenesis in Culex quinquefasciatus
Summary
This protocol describes microinjection procedures for Culex quinquefasciatus embryos that are optimized to work with CRISPR/Cas9 gene editing tools. This technique can efficiently generate site-specific, heritable, germline mutations that can be used for building genetic technologies in this understudied disease vector.
Abstract
Culex quinquefasciatus is a vector of a diverse range of vector-borne diseases such as avian malaria, West Nile virus (WNV), Japanese encephalitis, Eastern equine encephalitis, lymphatic filariasis, and Saint Louis encephalitis. Notably, avian malaria has played a major role in the extinction of numerous endemic island bird species, while WNV has become an important vector-borne disease in the United States. To gain further insight into C. quinquefasciatus biology and expand their genetic control toolbox, we need to develop more efficient and affordable methods for genome engineering in this species. However, some biological traits unique to Culex mosquitoes, particularly their egg rafts, have made it difficult to perform microinjection procedures required for genome engineering. To address these challenges, we have developed an optimized embryo microinjection protocol that focuses on mitigating the technical obstacles associated with the unique characteristics of Culex mosquitoes. These procedures demonstrate optimized methods for egg collection, egg raft separation and other handling procedures essential for successful microinjection in C. quinquefasciatus. When coupled with the CRISPR/Cas9 genome editing technology, these procedures allow us to achieve site-specific, efficient and heritable germline mutations, which are required to perform advanced genome engineering and develop genetic control technologies in this important, but currently understudied, disease vector.
Introduction
C. quinquefasciatus, commonly known as the southern house mosquito, is a competent vector of numerous pathogens including West Nile virus (WNV), Japanese encephalitis, Saint Louis encephalitis, and Eastern equine encephalitis. In particular, since it was first detected in New York in 1999, WNV has become a major vector-borne disease throughout the continental United States (US) with over 50,000 reported human cases resulting in around 2,300 deaths between 1999 and 20181 as well as over 4,500 reported equine cases between 2008-20192. In addition, at least 23 bird species found in North America have been impacted by WNV infections with at least 12 species classified as irrecoverable as a result of WNV3. The impact of WNV on human, equine, and avian populations is due to the opportunistic feeding behavior of its vectors. Typically, birds are the primary hosts for WNV, and humans and horses are incidental or dead-end hosts. Some pathogens vectored by C. quinquefasciatus only infect birds such as the avian malaria parasite, Plasmodium relictum. In Hawaii, C. quinquefasciatus is a principal vector of avian malaria and has caused the extinction of many native bird species4,5.
To control diseases transmitted by C. quinquefasciatus, researchers and vector control agencies have used commonly established mosquito population control tools such as insecticide application6, however, these methods are costly, not species-specific, and have limited effectiveness as resistance to insecticides is high in many C. quinquefasciatus populations6,7,8,9. Other control techniques, such as Wolbachia-based population control strategies have been developed in recent years10,11, but the fitness costs associated with Wolbachia infection limit the feasibility of this approach for this vector12. There are also genetic-based control methods that have been developed in other mosquito species such as Aedes aegypti13,14, Anopheles gambiae15 and Anopheles stephensi16, including the development of pathogen resistant mosquitoes17,18,19, that could also be developed for C. quinquefasciatus if the requisite genome engineering tools are developed for this species. However, C. quinquefasciatus biology differs greatly from other Aedes and Anopheles mosquito vectors which has made the development of similar genetic technologies difficult in this vector. With the advent of CRISPR-based genome engineering technologies, precise genome engineering has become increasingly trivial, affordable and adaptable and consequently has led to the development of novel genetic tools in a wide variety of species.
To generate mutations with CRISPR-based technologies, a mixture of Cas9 protein and synthetic guide RNA (sgRNA), complementary to the desired loci, is microinjected into pre-blastoderm stage embryos. Since C. quinquefasciatus females lay their eggs in groups attached in a floating raft structure (Figure 1), as opposed to ovipositing individual eggs, a trait of Aedes and Anopheles mosquitoes, embryo microinjections are increasingly complicated in this species. Culex larvae also emerge from the anterior side of each egg, which is in contact with the water surface (Figure 1), so egg orientation post manipulation is important in this species. Here we describe a detailed protocol designed for the microinjection of Cas9 protein and sgRNA into C. quinquefasciatus embryos. This protocol has been designed to accommodate traits unique to Culex biology in order to improve embryo survival and genome mutation rates through certain steps that are key for timely egg collection and egg survival.
Protocol
1. C. quinquefasciatus colony rearing
- Set up multiple colonies of C. quinquefasciatus adults in bug dorm cages.
NOTE: The colonies were provided by Dr. Laura Harrington at Cornell University20. Detailed protocols for rearing Culex mosquitoes can be found in other literature21.- Maintain the mosquitoes at 25 ± 1 ˚C at 30% humidity with a 12:12 h day:night cycle.
- Provide 20% sugar solution ad libitum by introducing a sugar solution container with a wick into the cage or by saturating cotton balls with the sugar solution and placing it within the cage.
- Allow mosquitoes to mate for at least 3 days prior to blood feeding (Figure 2A).
2. Collection of C. quinquefasciatus pre-blastoderm stage embryos
- After 3-6 days post eclosion, provide females with 1-2 mL of a citrated bovine blood-meal through a synthetic membrane and heated to approximately 40 °C in a blood feeding system (Figure 2B). There are also alternative blood feeding protocols that can be used for Culex mosquitoes (e.g., see ref21).
NOTE: C. quinquefasciatus is known for a preference for avian blood. Some labs use anesthetized live birds or avian blood for their blood meal source21,22,23. However, colonies can be trained/selected to feed on bovine blood or small rodents if this feature is selected for over multiple generations.- Allow females to rest for a minimum of 3 days after blood feeding to undergo oogenesis and egg maturation before inducing oviposition for microinjection experiments.
- On the day of embryonic microinjections, create an oviposition cup with organically infused oviposition water. Oviposition water should be made by fermenting rabbit feces (50 g/L), decomposing grass (4.5 g/L), or fish food (25 g/L) in water over the course of 5 or more days24,25.
- Place the oviposition cup into the cage and place the entire cage into a dark location (Figure 2C).
- After every 30 min, check the cup for egg rafts.
- If egg rafts are present, collect the rafts by scooping them with a paintbrush and place them on a wet filter paper (Figure 2D,E).
3. Alignment of C. quinquefasciatus pre-blastoderm stage embryos
- Separate the eggs from the rafts by pressing down on the raft and teasing apart the eggs individually using a fine tip paintbrush and forceps (Figure 2E).
- Align individual eggs on a thin strip of double-sided sticky tape placed across the top of a glass slide (Figure 2F).
- While aligning, try to point the anterior side of each egg in the same direction for easier accessibility.
NOTE: An alternative egg alignment method without double sided sticky tape can be found in a previously published Nasonia vitripennis embryo microinjection protocol26. - Cover eggs with the halocarbon oil mix.
NOTE: Halocarbon mix can be prepared ahead of time by gently mixing two halocarbon reagents and water (9:1:20, halocarbon 700 : halocarbon 27 : Ultrapure water) and then incubating the mixture overnight at 25οC to facilitate water saturation of the halocarbon oil.
4. Needle preparation for microinjections
- Generate the aluminosilicate capillary glass needles using a glass micropipette puller.
- Place an aluminosilicate capillary glass into a needle puller, as per instructions from the needle puller’s user manual.
NOTE: Quartz and borosilicate capillary needles can also be used, but for this experiment, aluminosilicate is preferred because of its relative affordability and durability. - Set Heat to 516, Velocity to 100, Delay to 70, Pull to 97, and Pressure to 500 on the needle puller.
- Activate the needle puller, as per the puller’s instructions and repeat as needed for additional needles.
NOTE: During the injection process, needles often become clogged or accidentally broken, therefore, pulling additional needles for a single experiment is highly recommended.
- Place an aluminosilicate capillary glass into a needle puller, as per instructions from the needle puller’s user manual.
- Bevel the needle tip by gently touching the tip of the pulled needle on the rotating diamond abrasive plate for around 10 s at a 50˚ angle.
NOTE: Beveling the needle opens it to allow fluid to flow through while also creating a sharper tip for easier penetration into the embryo. An example of a proper needle can be seen in a previous article26. - Store pulled and beveled needles by embedding them into lines of modeler’s clay in a Petri dish.
NOTE: To ensure the best quality for needle tips, needles should be freshly pulled and beveled as close to the time of injection as possible.
5. Loading the injection mixture
- Prepare the injection mixture consisting of genome modification reagents (e.g., 200 ng/μL sgRNA and 200 ng/μL Cas9 mixture), or preferred injection solutions and keep it on ice.
NOTE: This mix can be prepared while waiting for eggs to be laid. More details on Cas9 and sgRNA production and preparation for microinjection can be found in previous publications27,28,29,30. - Load 2 μL of injection mixture into the injection needle using a microloader tip.
6. Microinjection set up
- Place the filled injection needle into a micromanipulator set up linked to an electronic microinjector.
- Place the glass slide containing the aligned eggs on the stage of a compound microscope.
- Using the micromanipulator and compound microscope, align the needle to aim at the posterior end of the embryo at a 25-35˚ angle.
7. Embryo microinjection (Figure 2G)
- Carefully insert the needle into the embryo and inject the mixture at a quantity of about 10% of the volume of the embryo (700-800 pL depending on the size of the eggs).
NOTE: When injecting, the egg should slightly swell, however, if too much fluid is injected, eggs may burst, or cytoplasmic fluid may leak out. - Inject around 20 eggs at a time then stop and perform embryo recovery procedures.
NOTE: While injecting, there is a high chance of needles to either clog or break. If a clog occurs, which can be determined by a lack of injection fluid flowing through the needle, try either cleaning out the needle with the clean function on the electronic microinjector or try re-beveling the needle. If neither step works, quickly change to a new needle while ensuring the prepared eggs remain moistened.
8. Embryo recovery and hatching
- Leave the eggs undisturbed for at least 5 min. Within 20 min post-injection, carefully remove the halocarbon oil by brushing lightly with a clean paintbrush (Figure 2H).
- Lift the eggs gently using a paintbrush and place them into a cup of double-distilled water (Figure 2I). Take care to keep the eggs on the surface of the water.
- Check the eggs daily for 7 days for hatching (Figure 2J).
- Follow normal larval rearing procedures21.
9. Screening for genome modification
- Screen the injected mosquitoes for the mutant phenotypes using a stereoscope (Figure 2K).
- Verify mutations that do not have an easily recognizable phenotype, by PCR amplification, T7 Endonuclease I assay, and sequencing by subcloning of the target region31.
- Set up additional crosses between injected individuals to detect heritable mutations within the germline.
Representative Results
In previously published experiments this method was used to successfully generate somatic and germline mutations of a gene critical for the development of dark eye pigmentation, white (CPIJ005542)30 (Table 1). CRISPR/Cas9 generated somatic mutations were scored by screening for loss of pigmentation in pupal stage eyes of injected individuals (G0). Somatic mutations were generally present as mosaic phenotypes where some but not all the cells have the mutant phenotype. For example, somatic mutations of the white gene resulted in a mixture of ommatidia with phenotypes that lack pigmentation and those with a wildtype pigmented phenotype30. When there was a germline mutation of the white gene, however, the next generation (G1) inherited the full white eyed phenotype. Germline mutation rates were determined by intercrossing mosaic G0 individuals and scoring for completely white eyed offspring (G1). These experiments resulted in a 64% to 82% embryo survival rate, as well as a 37% to 57% somatic mutagenesis rate and a >61% germline mutagenesis rate (Table 1). By multiplexing sgRNAs to target multiple loci in the same gene, somatic and germline mutagenesis rates increased upwards to as much as 86% (Table 1). In addition, for many generations, viable homozygous stocks of the white mutants have been successfully kept in the lab.
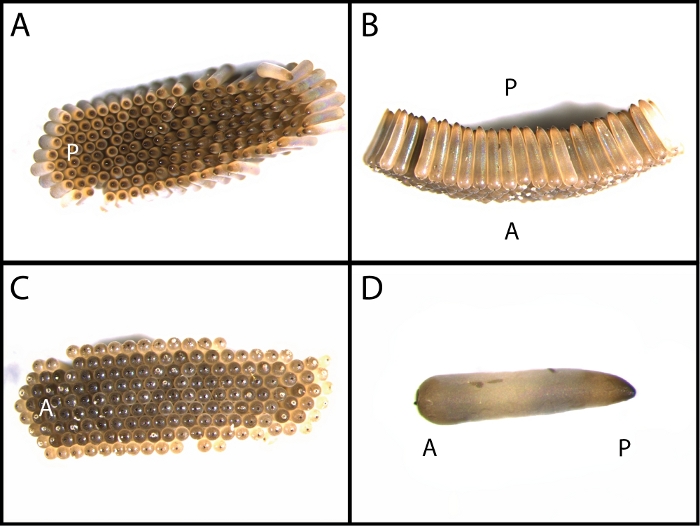
Figure 1: C. quinquefasciatus egg raft and single egg morphology.
Dorsal (A), lateral (B), and ventral (C) views of C quinquefasciatus egg rafts. C. quinquefasciatus females lay their eggs with the more bulbous anterior side touching the surface of the water while the more pointed posterior away from the water’s surface (D). A- anterior; P- posterior Please click here to view a larger version of this figure.

Figure 2: Timeline for creating C. quinquefasciatus mutants by microinjection.
Timeline of C. quinquefasciatus colony preparation and embryo collection for microinjection (A-D). Collected eggs are then separated (E) aligned in the same orientation and covered with halocarbon oil (F). The eggs are then injected (G) and allowed to rest for a minimum of 5 min before removing the halocarbon oil (H). Eggs are then transferred to a clean water source (I) for hatching (J) and screened for mutant phenotypes (K). Please click here to view a larger version of this figure.
| Survival | Somatic mosaicism (G0) | Germline mutagenesis (G1) | ||||||
| sgRNA | #injected | ♂ | ♀ | Total (%) | ♂ | ♀ | Total (%) | G1 mutants (%) |
| wsgRNA-1 | 50 | 15 | 20 | 35(70) | 7(47) | 13(65) | 20(57) | 128(69) |
| wsgRNA-2 | 50 | 9 | 32 | 41(82) | 3(33) | 12(38) | 15(37) | 51(61) |
| wsgRNA-3 | 50 | 17 | 15 | 32(64) | 7(41) | 8(53) | 15(47) | 157(72) |
| wsgRNA-1/wsgRNA-2 | 50 | 7 | 16 | 23(46) | 5(71) | 12(75) | 17(74) | 123(79) |
| wsgRNA-1/wsgRNA-3 | 50 | 13 | 9 | 22(44) | 10(77) | 6(67) | 16(73) | 72(81) |
| wsgRNA-2/wsgRNA-3 | 50 | 17 | 10 | 27(54) | 13(76) | 8(80) | 21(78) | 101(85) |
| wsgRNA-1/wsgRNA-2/wsgRNA-3 | 50 | 11 | 10 | 21(42) | 9(82) | 9(90) | 18(86) | 149(86) |
Table 1: Survival and mutation rates of embryos injected with single and multiplexed gRNAs targeting white. The table is reprinted with permission from30
Discussion
With recent efforts to generate genetically engineered tools for mosquito vector control, there is a need to develop and optimize embryo microinjection protocols for common mosquito disease vectors. Although methods have been developed for Aedes and Anopheles mosquitoes, a protocol designed specifically for Culex has been minimally explored. In general mosquito embryo injection protocols can be divided into 3 general steps: 1) embryo collection and preparation, 2) embryo injection, and 3) post-injection recovery. In order to successfully generate mutants, all three steps must be optimized for the target species. This modified protocol for embryo microinjection is specific for successful genome engineering of Culex mosquitoes.
Maximizing embryo collection is a common bottleneck in embryo microinjection protocols. In order to increase the number of eggs collected in a short period of time, mosquitoes were restricted from an oviposition substrate for 2-3 days post blood meal. It is important to note that C. quinquefasciatus tend to prefer avian blood sources as opposed to mammalian sources or membrane feeding with mammalian blood. However, many researchers have difficulty acquiring a reliable source of live avians or avian blood for blood feeding, so laboratory stocks can be adapted to feed on more accessible blood sources. It is also crucial to use nutrient-rich water for the oviposition substrate as it provides oviposition cues for C. quinquefasciatus and most other Culex vectors. There are many methods to generate nutrient-rich water, which may need to be optimized between labs to ensure an appropriate number of eggs are available for microinjection. For example, while this method was the most successful with rabbit feces fermented in deionized water for 5 or more days, other researchers have more success with grass or fish food as the nutrient source and some even with distilled water21.
Since Culex mosquitoes lay groups of eggs in rafts, collecting eggs is fairly simple. Eggs can simply be collected by scooping the entire raft with a paintbrush. Egg separation is more complicated, but essential for successful injection. Eggs can be carefully separated from the raft by applying gentle downward pressure between eggs with paintbrush or forceps. With some practice, multiple users were reliably able to separate single eggs from egg rafts. After separating each egg, the eggs are aligned in the same orientation on double-sided sticky tape, which stabilizes the eggs during microinjection. Eggs are injected into the tapered posterior end and the addition of halocarbon oil keeps the eggs moist during manipulation.
To limit embryo damage during microinjection, injection needles need to be of appropriate strength and beveled at an appropriate angle. Aluminosilicate needles beveled for 10 seconds at a 50˚ angle had the best results, but borosilicate and quartz needles may also work, even though they may be less durable and more expensive. In addition, proper needle beveling facilitates better flow of the Cas9 and sgRNA mixtures and creates a sharper point for easier penetration into the embryo. In many cases, beveling is also an efficient way to quickly fix clogged needles instead of spending the time and effort to replace clogged needles.
After injection, eggs should remain undisturbed for at least 5 minutes with the halocarbon oil being removed immediately afterward by gently brushing it away with a clean paintbrush. Following removal of the halocarbon oil, the injected eggs can be placed in water to hatch, which typically occurs within 3 days post-injection. With practice and sufficient numbers of eggs, this protocol can achieve consistent somatic and germline mutations in C. quinquefasciatus and is versatile enough that it should be easily adaptable to other Culex mosquitoes.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by UCSD startup funds directed to O.S.A.
Materials
| 9 oz clear plastic pet cup | Karat | C-KC9 | Insect Rearing and Egg Collection |
| Aluminosilicate glass capillary tubing 1mm(outside diameter) X 0.58mm (inner diameter) | Sutter Instruments | BF100-58-10 | Microinjection, borosilicate and quartz needles could also be used but we prefered aluminosilicate |
| Blood | Colorado Serum Company | 31025 | The colony took multiple generations to adapt to this blood source. Other blood sources are likely just as appropriate. See protocol notes on blood source selection. |
| Bugdorm | Bugdorm | 4F2222 | Insect Rearing Cage |
| Cas9 Protein with NLS | PNABio | CP01 | Microinjection |
| Compound Microscope | Olympus BX41 | Microinjection, for embryo injection | |
| Diamond abrasive plate (0.7u to 2.0u tip sizes) | Sutter Instruments | 104E | Microinjection, to be used with beveler |
| DNase/RNase-Free distilled Water | Invitrogen | 10977-015 | Microinjection |
| Double-sided Tape | Scotch | B084NVQGXD | Microinjection, embryo alignment |
| Femtojet 4x or 4i programmable microinjector | Eppendorf | Microinjection | |
| Femtotips Microloader tips | Fisher Scientific | E5242956003 | Microinjection |
| Filter Paper | Whatman | 1001-090 | Microinjection, embryo collection |
| Fine-tip paintbrush | ZEM | 2595 | Microinjection, embryo alignment |
| Halocarbon oil 27 | Sigma-Aldrich | H8773 | Microinjection, embryo alignment |
| Halocarbon oil 700 | Sigma-Aldrich | H8898 | Microinjection, embryo alignment |
| Hemotek | Hemotek | PS5 | Line Maintenance |
| Microelectrode Beveler | Sutter Instruments | BV10 | Microinjection, needle beveling |
| Micropipette Puller | Sutter Instruments | P-1000 or P-2000 | Microinjection, needle pulling |
| Microscope Slides | Fisherbrand | 12-550-A3 | Microinjection, embryo alignment |
| Non-Drying Modeling Clay | Jovi | B0025Z71IM | Microinjection, needle storage |
| Stereo Microscope | Olympus | SZ51 | Microinjection, for embryo alignment |
| Sugar | Domino | 20% sugar solution for adult sugar source. | |
| T7 Endonuclease I | NEB | M0302 | Preparation of microinjection materials |
| TOPO TA Cloning Kit | ThermoFischer Scientific | K451020 | Preparation of microinjection materials |
| Ultra-fine tip forceps | Fisher Scientific | 16-100-121 | Microinjection, embryo alignment |
References
- Final Cumulative Maps and Data | West Nile Virus. CDC Available from: https://www.cdc.gov/westnile/statsmaps/cumMapsData.html#eight (2019)
- APHIS | West Nile Virus (WNV). USDA Available from: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/equine/wnv (2020)
- Luke George, T., et al. Persistent impacts of West Nile virus on North American bird populations. Proceedings of the National Academy of Sciences of the United States of America. 112 (46), 14290-14294 (2015).
- van Riper, C., van Riper, S. G., Lee Goff, M., Laird, M. The Epizootiology and Ecological Significance of Malaria in Hawaiian Land Birds. Ecological Monographs. 56 (4), 327-344 (1986).
- Liao, W., Atkinson, C. T., LaPointe, D. A., Samuel, M. D. Mitigating Future Avian Malaria Threats to Hawaiian Forest Birds from Climate Change. PloS One. 12 (1), 0168880 (2017).
- Martins, W. F. S., et al. Transcriptomic analysis of insecticide resistance in the lymphatic filariasis vector Culex quinquefasciatus. Scientific Reports. 9 (1), 11406 (2019).
- Norris, L. C., Norris, D. E. Insecticide resistance in Culex quinquefasciatus mosquitoes after the introduction of insecticide-treated bed nets in Macha, Zambia. Journal of Vector Ecology: Journal of the Society for Vector Ecology. 36 (2), 411-420 (2011).
- Corbel, V., et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta tropica. 101 (3), 207-216 (2007).
- Yadouléton, A., et al. Insecticide resistance status in Culex quinquefasciatus in Benin. Parasites & Vectors. 8, 17 (2015).
- Atyame, C. M., et al. Wolbachia-based population control strategy targeting Culex quinquefasciatus mosquitoes proves efficient under semi-field conditions. PloS One. 10 (3), 0119288 (2015).
- Atyame, C. M., et al. Cytoplasmic incompatibility as a means of controlling Culex pipiens quinquefasciatus mosquito in the islands of the south-western Indian Ocean. PLoS Neglected Tropical Diseases. 5 (12), 1440 (2011).
- Almeida, F., et al. Effects of Wolbachia on fitness of Culex quinquefasciatus (Diptera; Culicidae). Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases. 11 (8), 2138-2143 (2011).
- Li, M., et al. Development of a confinable gene drive system in the human disease vector Aedes aegypti. eLife. 9, 51701 (2020).
- Phuc, H. K., et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biology. 5, 11 (2007).
- Kyrou, K., et al. A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nature Biotechnology. 36 (11), 1062-1066 (2018).
- Gantz, V. M., et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proceedings of the National Academy of Sciences of the United States of America. 112 (49), 6736-6743 (2015).
- Buchman, A., et al. Engineered resistance to Zika virus in transgenic expressing a polycistronic cluster of synthetic small RNAs. Proceedings of the National Academy of Sciences of the United States of America. 116 (9), 3656-3661 (2019).
- Buchman, A., et al. Broad dengue neutralization in mosquitoes expressing an engineered antibody. PLoS Pathogens. 16 (1), 1008103 (2020).
- Isaacs, A. T., et al. Engineered resistance to Plasmodium falciparum development in transgenic Anopheles stephensi. PLoS Pathogens. 7 (4), 1002017 (2011).
- Liu, N., Li, T., Reid, W. R., Yang, T., Zhang, L. Multiple Cytochrome P450 genes: their constitutive overexpression and permethrin induction in insecticide resistant mosquitoes, Culex quinquefasciatus. PloS One. 6 (8), 23403 (2011).
- Kauffman, E., et al. Rearing of Culex spp. and Aedes spp. Mosquitoes. Bio Protocols. 7 (17), 2542 (2017).
- Richards, S. L., Anderson, S. L., Yost, S. A. Effects of blood meal source on the reproduction of Culex pipiens quinquefasciatus (Diptera: Culicidae). Journal of Vector Ecology: Journal of the Society for Vector Ecology. 37 (1), 1 (2012).
- Kitzmiller, J. B., Micks, D. W. Techniques for Rearing Culex Mosquitoes. American Midland Naturalist. 52 (1), 253 (1954).
- Mordue, A. J., Blackwell, A., Hansson, B. S., Wadhams, L. J., Pickett, J. A. Behavioural and electrophysiological evaluation of oviposition attractants for Culex quinquefasciatus say (Diptera: Culicidae). Experientia. 48 (11-12), 1109-1111 (1992).
- Allgood, D. W., Yee, D. A. Oviposition preference and offspring performance in container breeding mosquitoes: evaluating the effects of organic compounds and laboratory colonisation. Ecological Entomology. 42 (4), 506-516 (2017).
- Li, M., Bui, M., Akbari, O. S. Embryo Microinjection and Transplantation Technique for Nasonia vitripennis Genome Manipulation. Journal of Visualized Experiments. (130), e56990 (2017).
- Li, M., et al. Generation of heritable germline mutations in the jewel wasp Nasonia vitripennis using CRISPR/Cas9. Scientific Reports. 7 (1), 1-7 (2017).
- Li, M., Akbari, O. S., White, B. J. Highly Efficient Site-Specific Mutagenesis in Malaria Mosquitoes Using CRISPR. Genes, Genomes, Genetics. 8 (2), 653-658 (2018).
- Li, M., Bui, M., Yang, T., White, B. J., Akbari, O. S. Germline Cas9 Expression Yields Highly Efficient Genome Engineering in a Major Worldwide Disease Vector, Aedes aegypti. Proceedings of the National Academy of Sciences of the United States of America. 114 (49), 10540-10549 (2017).
- Li, M., et al. Methods for the generation of heritable germline mutations in the disease vector Culex quinquefasciatus using clustered regularly interspaced short palindrome repeats-associated protein 9. Insect Molecular Biology. 29 (2), 214-220 (2020).
- New England Biolabs Determining Genome Targeting Efficiency using T7 Endonuclease I. NEB Available from: https://www.neb.com/protocols/2014/08/11/determining-genome-targeting-efficiency-using-t7-endonuclease-i (2020)

