Intrathecal Delivery of Antisense Oligonucleotides in the Rat Central Nervous System
Summary
Here, we describe a method for delivering drugs to the rat central nervous system by implanting a catheter into the lumbar intrathecal space of the spine. We focus on the delivery of antisense oligonucleotides, though this method is suitable for delivery of other therapeutic modalities as well.
Abstract
The blood brain barrier (BBB) is an important defense against the entrance of potentially toxic or pathogenic agents from the blood into the central nervous system (CNS). However, its existence also dramatically lowers the accessibility of systemically administered therapeutic agents to the CNS. One method to overcome this, is to inject those agents directly into the cerebrospinal fluid (CSF), thus bypassing the BBB. This can be done via implantation of a catheter for either continuous infusion using an osmotic pump, or for single bolus delivery. In this article, we describe a surgical protocol for delivery of CNS-targeting antisense oligonucleotides (ASOs) via a catheter implanted directly into the cauda equina space of the adult rat spine. As representative results, we show the efficacy of a single bolus ASO intrathecal (IT) injection via this catheterization system in knocking down the target RNA in different regions of the rat CNS. The procedure is safe, effective and does not require expensive equipment or surgical tools. The technique described here can be adapted to deliver drugs in other modalities as well.
Introduction
The vascular system of the central nervous system (CNS) has evolved as a critical regulator of homeostasis, controlling traffic of molecules, supplying nutrients and getting rid of waste. This system is also the first line of defense from attacks of external pathogens, thanks to a dense distribution of tight junctions along the walls of the endothelial cells. These tight junctions make up one aspect of the blood brain barrier (BBB). While the BBB allows the transport of molecules required to fulfill nutrient and energy demands (e.g., ions, glucose), it also selectively limits the passage of pathogens as well as toxic chemicals1,2,3.
Ironically, the same protective function of the BBB that limits passage of pathogens and toxic chemicals also is the major obstacle to our ability to easily access the CNS with therapeutic treatments after systemic administration to the organism2,4,5. This role of the BBB has prompted the development of a plethora of new drug distribution technologies and approaches6.
One way to overcome this obstacle is to inject the drugs directly into the cerebrospinal fluid (CSF) that continuously perfuses both the brain and spinal cord7,8,9,10. In this article, we describe a method to successfully deliver agents into the lumbar intrathecal space by placing the internal end of the catheter completely in the cauda equina space of the rat spine. A description of this procedure was previously published by Mazur et al. elsewhere11.
The protocol is very effective and produces a greater than 90% success rate of antisense oligonucleotide (ASO) delivery to the CNS when assessed by quantitative polymerase chain reaction (qPCR) analysis of target gene knockdown8. The procedure causes minimal discomfort to the animals, as 100% of the rats survive the surgery and show minimal swelling around the surgical wound and no signs of distress (e.g., hyperactivity, dehydration, circling, loss of balance, decreased food intake, and dehydration) during post-op observation. Another advantage of the method described here is that it does not require expensive equipment, nor any special tools.
Protocol
All in vivo procedures were performed under Biogen Institutional Animal Use and Care Committee (IACUC) approved protocols which follow the guidelines set forth by the United States National Institutes of Health guide for the care and use of laboratory animals.
1. Material and instrument preparation
- Prepare the special guide cannulas.
- Use a rotary tool with cut-off wheel (or a sharp saw) to cut off the two ends of a 19 G needle, resulting in a ~1.5−2 cm long guide cannula (Figure 1Aiii). Use the grinding wheel of the rotary tool to smooth the two ends.
NOTE: Alternatively, premade and sterile guide cannulas can be purchased from a commercial vendor (Table of Materials).
- Use a rotary tool with cut-off wheel (or a sharp saw) to cut off the two ends of a 19 G needle, resulting in a ~1.5−2 cm long guide cannula (Figure 1Aiii). Use the grinding wheel of the rotary tool to smooth the two ends.
- Prepare the catheter/wire assembly.
- Cut an 8 cm long piece of PE-10 tubing (polyethylene tubing, diameter 0.011 inch) to serve as the intrathecal catheter. Make a mark 2 cm from one end with an ethanol resistant marker pen. Cut an 11 cm long stylet wire from polytetrafluoroethylene coated stainless steel wire. Insert the stylet wire (Figure 1Aii) into the lumen of the PE-10 catheter (Figure 1Ai).
NOTE: One catheter/wire assembly set (Figure 1Av) is needed for each animal. Alternatively, catheters and stylet wires can be purchased from a commercial vendor (Table of Materials).
- Cut an 8 cm long piece of PE-10 tubing (polyethylene tubing, diameter 0.011 inch) to serve as the intrathecal catheter. Make a mark 2 cm from one end with an ethanol resistant marker pen. Cut an 11 cm long stylet wire from polytetrafluoroethylene coated stainless steel wire. Insert the stylet wire (Figure 1Aii) into the lumen of the PE-10 catheter (Figure 1Ai).
- Prepare delivery catheter assembly.
- Cut a piece of PE-50 catheter (5−10 cm, polyethylene tubing, diameter 0.023 inch) (Figure 1Bi). To one end of the PE50 catheter insert a 23 G tubing adaptor (Figure 1Biv). This end will be connected to a 100 µL syringe (Table of Materials) during surgery. Insert a modified 30 G needle with hub cut off (Figure 1Biii) into an approximately 0.5 cm piece of PE-10 tubing (Figure 1Bii) and connect the PE-10 tubing to the other end of the catheter.
- The 30 G needle end of the delivery catheter assembly (Figure 1Bv) will be connected to the distal end of the implanted PE-10 catheter in the rat during surgery.
NOTE: Alternatively, the delivery catheter assembly (Figure 1Bv) can be purchased from a commercial vendor (Table of Materials).
- Prepare the guide cannula-needle assembly (Figure 1Avi) by placing a guide cannula (Figure 1Aiii) over the end of a 23 G needle.
- Sterilize all surgical instruments including the guide cannula and the wire/catheter sets using an ethylene oxide sterilizer for 12 h.
NOTE: All surgical instruments except the catheter can be autoclaved; catheter will melt at high temperature.
2. Surgery preparation
NOTE: This procedure is routinely performed on male and female Sprague Dawley rats with body weights between 200 g and 400 g. Two rats are housed per cage under a 12 h light/dark cycle with free access to food and water.
- Weight a rat and place it in an isoflurane chamber to induce anesthesia (1−5% isoflurane in O2, titrated to effect).
NOTE: The rat is then continuously anesthetized with isoflurane to maintain deep anesthesia via a nose cone throughout the procedure. An alternative anesthesia method (e.g., administration of ketamine 100 mg/kg and xylazine 10 mg/kg) can be used as approved by the IACUC. - When the rat fails to respond to a toe pinch, shave its back from the tail to the caudal thoracic spine and place the shaved rat on the a sterile sheet laid on top of a heating pad.
- Position a 50 ml conical centrifuge tube under the abdomen of the rat to flex the spine in the lumbar region (Figure 2A) and apply ophthalmic ointment to the eyes.
- Inject sustained release buprenorphine (1.0 mg/kg; Table of Materials) subcutaneously in the rat. Clean the exposed skin with alternating povidone and alcohol scrubs and repeat this three times.
NOTE: An alternative pain relief can be used as approved by the IACUC protocol. - Drape the animal with a sterile transparent drape that has been fenestrated over the surgical site.
3. Surgery
- With the rat supported by the 50 mL conical tube, identify the two natural pits between muscles above the pelvis (arrows in Figure 2A). With one hand holding those pits, use the other hand to gently press and feel the spine from caudal to rostral direction and find the first major indentation between vertebrae and this is the intervertebral space between S1 and L6 vertebrae (Figure 2B).
- Move slightly rostrally to identify the next indentation, the intervertebral space between the L5 and L6 vertebrae and the injection site (* in Figure 2A). Use a scalpel to make an incision no more than 2 cm long in the skin along the midline from rostral to caudal so that the injection site is at the center of the incision (dotted line in Figure 2A).
- Use dissection scissors to dissect away the connective tissue in order to visualize the muscle layer. Then make a 1 cm incision in the muscle capsule immediately lateral to the dorsal spinal process of the L6 lumbar vertebra.
NOTE: The bones of the L6 lumbar vertebra could be visualized at this point. - Position the guide cannula-needle assembly near the anterior aspect of the 6th lumbar vertebra and push it into the intervertebral space along the anterior aspect of the 6th vertebra so that the end of the needle penetrates the spinal canal. Push the guide cannula in place along the needle and remove the 23 G needle leaving only the guide cannula in place.
NOTE: It is helpful to use blunt forceps to locate the dorsal process of the L6 lumbar vertebra before inserting the needle. Generally, CSF fluid can be seen entering the needle hub (this fluid may be tinged with a hint of blood, but this does not indicate that harm has been done or that the needle is not placed correctly). The authors have not seen large amount of blood or severe bleeding during this procedure. If either occurs, a veterinarian should be contacted to determine the appropriate treatment and if animals should be euthanized. - Insert the catheter-wire assembly into the guide cannula. Angle down the catheter-wire assembly at approximately a 45° angle to the spinal canal and force the end approximately 0.3 cm into the spinal canal.
- Remove the guide cannula, leaving the catheter with the stylet wire in place. Remove the stylet wire approximately 2.5 cm from the intrathecal tip of the catheter and advance the catheter into the spinal canal until the 2 cm mark is at the entrance of the canal (just visible below the muscle) as shown in Figure 1Bvi.
NOTE: The inserted catheter should extend rostrally into the subarachnoid space. Successful placement should allow for free movement of the catheter in that space. - Completely withdraw the stylet wire, and CSF may be seen entering the implanted catheter.
- Connect the delivery catheter assembly to the distal end of the implanted catheter via the 30 G needle end (Figure 1Bv,vi).
- Load 60 µL of sterile saline into a 100 µL syringe (flushing syringe). Load a bolus of 30 µL of the test compound (e.g., ASO solution) into a second syringe (injection syringe).
- Connect the flushing syringe (loaded with saline) to the tubing adaptor end of the delivery catheter assembly (Figure 1Bv). Inject 20 µL of sterile saline into the intrathecal space (pre-injection flushing).
- Connect the injection syringe (loaded with test compound) to the tubing adaptor end of the delivery catheter assembly (Figure 1Bv). Inject 30 µL of test compound into the intrathecal space over 30 s.
NOTE: The routine injection volume of ASO is 30 µL to achieve good knockdown in the spinal cord and in the cortex. It has been reported that the injection volumes may impact compound distribution12, though different injection volumes have not been tested. If other compound or volume is used, the safety and effectiveness need to be empirically determined. - Repeat step 3.10 and flush the catheter with another 40 µL of sterile saline (post-injection flushing). Then detach the delivery catheter assembly from the implanted catheter.
NOTE: The pre and post-injection flush is thought to reduce local sequestration of the compounds and improve their distribution to the rostral structures12. - Aseptically cut and heat seal the implanted catheter: Place a pair of sterile dissection forceps in a bead sterilizer until they are very hot, then clamp down on the tubing with the hot end of the forceps.
NOTE: This action melts the catheter. Thus, the hole in the tube is collapsed and all sides stick to each other, sealing the tubing in an aseptic fashion and then it is placed into the subcutaneous space. - Use absorbable monofilament sutures to secure the remaining heat-sealed catheter to the connective tissue. Then use non-absorbable monofilament sutures to close the skin over the secured heat-sealed catheter.
NOTE: Wound clips can also be used as approved by the IACUC protocol. The technique is compatible with repeated injections though it has only been used for one-time injections in our hands. The feasibility of repeated injections should be empirically evaluated with the approval of the IACUC. - Use gauze and saline to wash any blood from the skin and allow the animal to recover from the anesthesia in a heated incubator until mobile, at which point it is returned to its home cage (two rats per cage).
NOTE: When performing surgeries on multiple rats on the same day, clean tools using water to remove blood and re-sterilize using a heated dry bead sterilizer (for at least 20 s, with time to cool) between animals. A new set of instruments is used every 5 animals. - Monitor the animals daily for at least 3 days after surgery and continue to monitor the animals weekly after recovering from surgery according to the IACUC protocol.
NOTE: If any complications occur (urine retention, incision infections, neurological disorder such as seizure or paralysis), a veterinarian should be contacted to determine the appropriate treatment and if animals should be euthanized. If sustained release buprenorphine is not used, pain relief should be given daily after surgery according to the IACUC protocol.
4. Evaluation of tissue specific knockdown after IT injection
- Two weeks after IT bolus injection of ASOs, collect different regions of the brain (i.e., cerebral cortex, striatum and cerebellum) as well as different segments of the spinal cord (i.e., cervical, thoracic, and lumbar). Extract total tissue RNA using a commercial RNA extraction kit and perform cDNA synthesis reaction as described previously13.
NOTE: Standard reagents were used for qPCR with the following assays: rat Malat1 and rat GAPDH. The relative transcript levels were calculated using the 2-ΔΔCT method (CT = threshold cycle).
Representative Results
Using the method described here, we injected two groups of adult female rats (250−300 g; n = 10/group) with either a single bolus of phosphate-buffered saline PBS or 300 µg of ASO targeting the long non-coding (linc) RNA Malat1; in our lab we routinely use the Malat1 ASO as a tool compound, because Malat1 is expressed ubiquitously and at high levels in all tissues14, including brain and spinal cord. The Malat1 ASO works via an RNaseH1-mediated mechanism15 that degrades the RNA, leading to knockdown (KD). In the experiment described here, we collected different regions of the brain (i.e., cerebral cortex, striatum and cerebellum) as well as the lumbar segment of the spinal cord, two weeks after delivery of the ASO. RNA from each of the collected region was then extracted and analyzed via qPCR, to assess the levels of expression of the Malat1 RNA.
When the tested agent is an ASO, we recommend to: 1) always collect multiple regions of the CNS, in order to compare ASO efficacy; 2) given the technical complexity of the surgical method, we recommend to include a positive control group, where a compound with well-established pharmacokinetic and pharmacodynamic properties (i.e., Malat1 ASO in our lab) is tested in parallel to the test agent; this will provide information on the effectiveness of the surgeries, should unexpected or unexplainable results be obtained (e.g., lack of or insufficient RNA regulation).
In the experiment described here, we obtained very good KD in all regions collected, as shown in Figure 3. However, we did observe some degree of regional variability with the spinal cord showing the highest percentage of KD (cerebral cortex = 87% KD, striatum = 77% KD, cerebellum = 74% KD, spinal cord = 94% KD). We have not accessed in vivo knockdown efficiency earlier than 2 weeks post-surgery. In our experiences with several ASOs, we detected significant knockdown of the target genes up to 6−8 weeks post-surgery (data not shown). A time-course study should be carried out if the precise time-dependent knockdown efficiency of a given ASO is of interest.
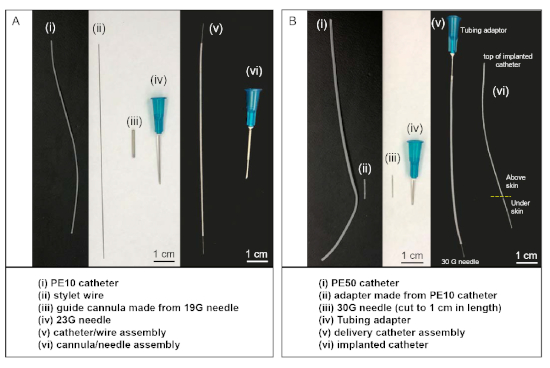
Figure 1: Customized material and catheter sets used in intrathecal injections. (A) The catheter/wire assembly (v) is made by inserting stylet wire (ii) into the lumen of PE-10 catheter (i). The cannula/needle assembly (vi) is made by inserting a 23 G needle into the lumen of the guide cannula (iii). (B) The delivery catheter assembly (v) is made by connecting tubing adapter to one end of the PE-50 catheter and connecting the cut 30 G needle into the other end using a piece of PE-10 catheter (ii) as an adaptor. During the surgery, the 30 G needle end of the delivery catheter assembly (v) is connected to the top of the implanted catheter (vi), after the other end of the implanted catheter is inserted into the intrathecal space of the animal. Please click here to view a larger version of this figure.
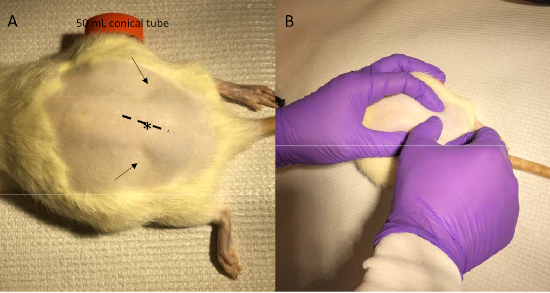
Figure 2: Identification of injection site and incision line. (A) With the abdominal of the rat supported by a 50 mL conical tube, the two pits between muscles above the pelvis are easily seen (arrows). (B) With one hand holding the pits, use the other hand to gently press and feel the spine and find the intervertebral space between the L5 and L6 vertebrae, i.e., the injection site (* in panel A). The dotted line in panel A shows the incision line with the injection site at its center. Please click here to view a larger version of this figure.
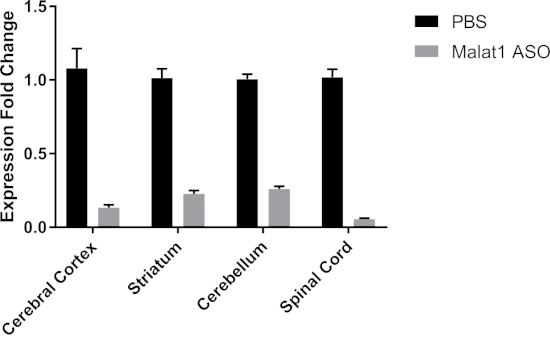
Figure 3: A single bolus IT injection of ASOs reduces rat Malat1 in vivo. We injected a single bolus of either PBS or 300 µg of Malat1 ASO; two weeks after the surgery we collected different regions of the CNS and quantified the expression levels of Malat1 RNA. We obtained good KD of Malat1 RNA in all regions analyzed, with some variability among regions (cerebral cortex = 87% KD, striatum = 77% KD, cerebellum = 74% KD, spinal cord = 94% KD; error bars = ± SEM). Please click here to view a larger version of this figure.
Discussion
The present article shows a powerful method to deliver therapeutic agents directly into the rat CNS. In theory, a similar technique can be also performed in mice, though due to the smaller size, the method can be more challenging. Therefore, our group performs intracerebroventricular (ICV) injections in mice for CNS drug delivery, which reach the same goals through a different route of administration. That method has been described in another study16.
The advantage of the method described here is that it does not require expensive equipment, nor any special tools. We recommend preparing the catheter/wire assembly shown in Figure 1A ahead of time. One should prepare at least as many catheter/wire assemblies as there are rats in the study, although we suggest preparing some extra catheter/wire assemblies in case some are damaged or need to be replaced during the surgery.
Once the technique is mastered, the whole procedure described requires about 25 min per rat, thus allowing treatment of many rats within one day. If one person performs the surgery on the first rat, and a second person does surgical preparation on the next rat, two rats can be processed at the same time to reduce per animal time. For a proficient operator, there is still a small chance for needle to reach epidural space instead of subarachnoid space. Observation of CSF backflow is a good indicator of correct needle position but it is not perfect. We recommend that a target engagement assay like the RNA knockdown analysis described in the result session should be performed to confirm correct delivery of the test compound.
Establishing techniques such as the one described here, is crucial for the development of a robust pre-clinical research pipeline that can advance CNS-targeting therapies. Indeed, IT delivery of ASOs as a therapeutic intervention is a method that is currently being explored for treatment of many disorders of the CNS17,18,19,20. Nusinersen, an ASO-based treatment for spinal muscular atrophy (SMA) patients, was recently approved in several markets worldwide, demonstrating the applicability of this method also to pediatric patients21,22,23.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Ionis Pharmaceuticals for supplying the ASOs described in the article.
Materials
| 3M Steri-Drape Small Drape with Adhesive Aperture | 3M | 1020 | ||
| 70% ethanol | Decon Laboratories, Inc | 8416-160Z | ||
| Alcohol swab sticks | Dynarex | NO 1204 | ||
| BD General Use Syringes 1 mL Luer-Lok tip | BD | 1ml TB Luer-Lok tip | BD 302830 | |
| BD Intramedic PE Tubing | BD | Polyethylene tubing PE50 Diameter 0.023 in | BD 427400 (10ft, Fischer Scientific 22-204008) or 427401 (100ft, Fischer Scientific 14-170-12P) | |
| BD Intramedic PE Tubing | BD | Polyethylene tubing PE10 Diameter 0.011 in | BD 427410 (10ft, Fischer Scientific 14-170-11B) or 4274011 (100ft, Fischer Scientific 14-170-12B) | |
| BD Intramedic PE Tubing Adapters | BD | 23 gauge intramedic luer stub adaper | BD 427565 or Fisher Scientific 14-826-19E | 120V 1.2A |
| BD PrecisionGlide Single-use Needles 30G | BD | BD 305128 | ||
| Buprenorphine Sustained Release-lab | ZooPharm | Prescription required | ||
| Ethylene oxide sterilizer | Andersen Sterilizer INC. | AN 74i, gas sterilizer | AN 74i | |
| Guide cannula | BD | 19G x 1 WT (1.1 mm x 25mm) needle | BD 305186 | |
| Hamilton syringe 100ul | Hamilton company | Hamilton syringe 100ul | ||
| Hot bead Sterilizer | Fine Science Tools | STERILIZER MODELNO FST 250 | ||
| Ophthalmic ointment | Dechra veterranery product | 17033-211-38 | ||
| Pocket Pro Pet Trimmer | Braintree Scientific | CLP-9931 B | ||
| Povidone scrub | PDI | S48050 | ||
| Saline | Baxter | Sodium Chloride 0.9% Intravenous Infusion BP 50ml | FE1306G | |
| Scalpel | Feather | disposable scalpel | No. 10 | |
| Small animal heating pad | K&H Manufacturing | Model # 1060 | ||
| Stylet Wire | McMaster-Carr | 1749T14 | LH-36233780 | |
| Surgery Towel drape | Dynarex | 4410 | ||
| Surgical scissors and forceps | FST and Fisher Scientific | |||
| Sutures | Ethicon | 4-0 or 5-0 | ||
| Tool to make the Guide cannular | Grainger | Rotary tool (Dremel) | 14H446 (Mfr: EZ456) | 1.5” diameter, Pk5 |
| EZ lock cut off Wheel | 1PKX5 (Mfr: 3000-1/24) | 1.5”, Pk2 | ||
| Grinding Wheel, Aluminum Oxide | 38EY44 (Mfr: EZ541GR) | |||
| EZ lock Mandrel | 1PKX8 (Mfr: EZ402-01) | 1.5” diameter | ||
| Diamond wheel floor Tile | 3DRN4 (Mfr: EZ545) | |||
| Alternative source for pre-made and sterilized materials for this procedure | ||||
| Dosing catheter system | SAI Infusion Systems | RIDC-01 | ||
| Guide cannula | SAI Infusion Systems | RIDC-GCA | ||
| Internal Catheters | SAI Infusion Systems | RIDC-INC | ||
| Stylet Wire | SAI Infusion Systems | RIDC-STY |
References
- Abbott, N. J. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cellular and Molecular Neurobiology. 25 (1), 5-23 (2005).
- Greene, C., Campbell, M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers. 4 (1), e1138017 (2016).
- Daneman, R., Engelhardt, B. Brain barriers in health and disease. Neurobiology of Disease. 107, 1-3 (2017).
- Ballabh, P., Braun, A., Nedergaard, M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiology of Disease. 16 (1), 1-13 (2004).
- Cardoso, F. L., Brites, D., Brito, M. A. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Research Reviews. 64 (2), 328-363 (2010).
- Larsen, J. M., Martin, D. R., Byrne, M. E. Recent advances in delivery through the blood-brain barrier. Current Topics in Medicinal Chemistry. 14 (9), 1148-1160 (2014).
- Brinker, T., Stopa, E., Morrison, J., Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 11, 10 (2014).
- Standifer, K. M., Chien, C. C., Wahlestedt, C., Brown, G. P., Pasternak, G. W. Selective loss of delta opioid analgesia and binding by antisense oligodeoxynucleotides to a delta opioid receptor. Neuron. 12 (4), 805-810 (1994).
- Wahlestedt, C., et al. Antisense oligodeoxynucleotides to NMDA-R1 receptor channel protect cortical neurons from excitotoxicity and reduce focal ischaemic infarctions. Nature. 363 (6426), 260-263 (1993).
- Wahlestedt, C., Pich, E. M., Koob, G. F., Yee, F., Heilig, M. Modulation of anxiety and neuropeptide Y-Y1 receptors by antisense oligodeoxynucleotides. Science. 259 (5094), 528-531 (1993).
- Mazur, C., et al. Development of a simple, rapid, and robust intrathecal catheterization method in the rat. Journal of Neuroscience Methods. 280, 36-46 (2017).
- Wolf, D. A., et al. Dynamic dual-isotope molecular imaging elucidates principles for optimizing intrathecal drug delivery. Journal of Clinical Investigation Insight. 1 (2), e85311 (2016).
- Becker, L. A., et al. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature. 544 (7650), 367-371 (2017).
- Zhang, X., Hamblin, M. H., Yin, K. J. The long noncoding RNA Malat1: Its physiological and pathophysiological functions. RNA Biology. 14 (12), 1705-1714 (2017).
- Crooke, S. T., Witztum, J. L., Bennett, C. F., Baker, B. F. RNA-Targeted Therapeutics. Cell Metabolism. 27 (4), 714-739 (2018).
- DeVos, S. L., Miller, T. M. Direct intraventricular delivery of drugs to the rodent central nervous system. Journal of Visualized Experiments. (75), e50326 (2013).
- McCampbell, A., et al. Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. Journal of Clinical Investigation. 128 (8), 3558-3567 (2018).
- Schoch, K. M., Miller, T. M. Antisense Oligonucleotides: Translation from Mouse Models to Human Neurodegenerative Diseases. Neuron. 94 (6), 1056-1070 (2017).
- Lane, R. M., et al. Translating Antisense Technology into a Treatment for Huntington’s Disease. Methods in Molecular Biology. 1780, 497-523 (2018).
- Wurster, C. D., Ludolph, A. C. Antisense oligonucleotides in neurological disorders. Therapeutic Advances in Neurological Disorders. 11, (2018).
- Haché, M., et al. Intrathecal Injections in Children With Spinal Muscular Atrophy: Nusinersen Clinical Trial Experience. Journal of Child Neurology. 31 (7), 899-906 (2016).
- Goodkey, K., Aslesh, T., Maruyama, R., Yokota, T. Nusinersen in the Treatment of Spinal Muscular Atrophy. Methods in Molecular Biology. 1828, 69-76 (2018).
- Wurster, C. D., Ludolph, A. C. Nusinersen for spinal muscular atrophy. Therapeutic Advances in Neurological Disorders. 11, (2018).

