Inducing Meningococcal Meningitis Serogroup C in Mice via Intracisternal Delivery
Summary
Here, we describe a method to induce meningococcal meningitis through an intracisternal route of infection in adult mice. We present a step by step protocol of meningococcal infection from the preparation of inoculum to the intracisternal infection; then record the animal survival and evaluate the bacterial loads in murine tissues.
Abstract
Neisseria meningitidis (meningococcus) is a narrow-host-range microorganism, globally recognized as the leading cause of bacterial meningitis. Meningococcus is a transient colonizer of human nasopharynx of approximately 10% of healthy subject. In particular circumstances, it acquires an invasive ability to penetrate the mucosal barrier and invades the bloodstream causing septicaemia. In the latest case, fulminating sepsis could arise even without the consequent development of meningitis. Conversely, bacteria could poorly multiply in the bloodstream, cross the blood brain barrier, reach the central nervous system, leading to fulminant meningitis. The murine models of bacterial meningitis represent a useful tool to investigate the host-pathogen interactions and to analyze the pathogenetic mechanisms responsible for this lethal disease. Although, several experimental model systems have been evaluated over the last decades, none of these were able to reproduce the characteristic pathological events of meningococcal disease. In this experimental protocol, we describe a detailed procedure for the induction of meningococcal meningitis in a mouse model based on the intracisternal inoculation of bacteria. The peculiar signs of human meningitis were recorded in the murine host through the assessment of clinical parameters (e.g., temperature, body weight), evaluation of survival rate, microbiological analysis and histological examination of brain injury. When using intracisternal (i.cist.) inoculum, meningococci complete delivery directly into cisterna magna, leading to a very efficient meningococcal replication in the brain tissue. A 1,000-fold increase of viable count of bacteria is observed in about 18 h. Moreover, meningococci are also found in the spleen, and liver of infected mice, suggesting that the liver may represent a target organ for meningococcal replication.
Introduction
Neisseria meningitidis is a Gram negative β-proteobacterium restricted to the human host, well known for being one of the most common causes of meningitis and sepsis in the human population across the world. It colonizes the upper respiratory tract (nose and throat) of healthy and asymptomatic carriers (2-30% of the population), but the bacterium sometimes evades various host immune defenses and spreads from the bloodstream to the brain causing an uncontrolled local inflammation, known as meningococcal meningitis. A combination of host and bacterial factors appears to contribute to the transition from the commensal to the invasive behavior1.
N. meningitidis is specialized exclusively in human colonization and infection. It has a narrow host range and, therefore, has limited in vivo pathogenesis studies due to the lack of suitable animal models that reproduce the human meningococcal disease. As a result, it had led to fundamental gaps in the comprehension concerning the pathogenesis of septicemia and meningitis caused by meningococcus. In the last decades, the development of many in vitro systems allowed the identification of several meningococcal virulence factors2,3,4. Although these valuable studies provided important insights to understand the role of these factors for a successful meningococcal infection, these models did not allow assessment of the consequences of bacterial interactions with the humoral and cellular immune system and even less with the whole tissue. In vivo animal models of infection are of great relevance as well for the evaluation of protection degree conferred by vaccine formulations. As a human-tropic pathogen, meningococcus possess appropriate determinants necessary for successful infection such as surface structures (i.e., type IV pili and opacity proteins) and iron uptake systems for human receptors and transport proteins (i.e., transferrin and lactoferrin)5,6,7 to properly adhere, survive and invade the human host. Finally, the genetic variation abilities of the pathogen to evade and/or block the human immune response further contribute to the high species tropism8,9. Therefore, the absence of specific host factors, involved in the interaction, can block steps of the pathogen’s life cycle, establishing significant difficulties in the development of small animal models summarizing the meningococcal life cycle.
Over the past decades, several approaches have been developed to improve our understanding of the meningococcal infectious cycle. Infections of two animal model, mouse and rat, either intraperitoneally (i.p.) or intranasally (i.n.), were developed to reproduce meningococcal disease10,11,12,13,14,15,16,17. The laboratory mouse is probably one of the more versatile animals for inducing experimental meningococcal infection.
However, the i.p. way of infection leads to the development of severe sepsis although it does not mimic the natural route of infection, whereas the i.n. route of infection was useful to evaluate meningococcal pathogenesis, even though it may induce lung infection prior to sepsis10,11,12,13,14,15,16,17.
The i.p. mouse model was instrumental to assess the protection from the meningococcal challenge10,11,12. The mouse model of meningococcal colonization based on the i.n. route of infection has been developed with infant mice, as they are more susceptible to meningococci, to reproduce an invasive infection mimicking the course of the meningococcal disease in humans13,14,15,16,17. Moreover, to promote meningococcal replication in the murine host, a growing number of technical strategies were also applied including the administration of the iron to the animals to improve the infection, the use of high bacterial inoculum, mouse-passaged bacterial strain as well as the employment of infant or immunocompromised animal hosts10,13,15,18,19. Expression of specific human factors like CD4620 or transferrin21 has increased the susceptibility of mice to this human-tropic bacterium; the employment of the human skin xenograft model of infection has also been useful to evaluate the adhesion ability of meningococci to human endothelium22,23. Collectively, the recent development of humanized transgenic mice has improved the understanding of the meningococcal pathogenesis and its host interactions.
Previously, we developed a murine model of meningococcal meningitis where the inoculation of bacteria was performed into the cisterna magna of adult mice with mouse-passaged bacteria24. Clinical parameters and the survival rate of infected mice demonstrated the establishment of meningitis with characteristics comparable to those seen in the human host, as well as, the microbiological and histological analyses of the brain. From these infected mice, bacteria were, also, recovered from blood, liver, and spleen, and bacterial loads from peripheral organs correlated with the infectious dose. In particular, this model was employed to evaluate the virulence of an isogenic mutant strain defective in the L-glutamate transporter GltT24. Recently, using our mouse model of meningococcal meningitis based on i.cist. route with serogroup C strain 93/42862,24 and an isogenic mutant defective in cssA gene encoding for UDP-N-acetylglucosamine 2-epimerase25, we have analyzed the role of exposed sialic acid in the establishment of disease in mice.
In this protocol, we describe a straightforward method to induce experimental meningococcal meningitis based on the i.cist. route of infection in Balb/c adult mice. This method is particularly useful for the characterization of meningococcal infection in a murine host, as well as for the assessment of the virulence between wild type reference strains and isogenic mutants. The intra-cisternal route of infection ensures complete delivery of the meningococci directly into the cisterna magna, which in turn facilitates bacterial replication in the cerebrospinal fluid (CSF) and induces meningitis with features that mimic those present in humans2,24,25,26.
Protocol
This protocol was conducted to minimize animal suffering and reduce the number of mice in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC). In vivo experiments reported in this study were approved by the Ethical Animal Care and Use Committee (Prot. number 2, 14 December 2012) and the Italian Ministry of Health (Prot. number 0000094-A-03/01/2013). All the procedures should be performed inside the Biosafety Cabinet 2 (BSC2) in a BSL2 room, and the potential infected waste should be disposed in dedicated containers.
1. Infection of Mice with N. meningitidis Serogroup C Strain
CAUTION: N. meningitidis is potentially a harmful pathogen and all necessary precautions must be taken when handling this microorganism. The entire experimentation requires Biosafety Level 2 (BSL2) containment. The researcher involved in animal studies should wear disposable personal protective equipment (PPE) for the duration of the experiment.
- Preparation of bacteria for in vivo infection studies
- Pick a single colony from a fresh Neisseria meningitidis culture on GC (Gonococcal) agar plate supplemented with 1% (vol/vol) Polyvitox supplement and inoculate in 10 mL of GC broth.
- Grow the bacteria at 37 °C in an orbital shaker incubator with the speed of 220 rpm. Keep checking the O.D. of the culture with a spectrophotometer. Grow the culture until the early exponential phase at an optical density OD600nm of 0.7, corresponding to ≈ 7 x 108 CFU/mL.
- Once the required O.D. is obtained, make frozen stocks by adding 10% glycerol. Dispense 1 mL of the culture in the cryovials. Store the vials at -80 °C until use.
NOTE: Although it is better to use fresh bacterial culture, frozen stocks were used to simplify and standardize the in vivo experiment. Usually the frozen stocks have been employed within approximately 6 months from the preparation. - Before the infection, thaw frozen bacteria at room temperature.
- Harvest the bacterial cells by centrifugation for 15 min at 1,500 x g and resuspend in 1 mL of fresh GC broth containing iron dextran (5 mg/kg).
NOTE: The GC broth is prepared with the addition of iron dextran (5 mg/kg) in order to favor the replication of meningococci in the host tissue14,18,27. - Before using, perform viable counts of bacteria to determine the exact number of CFU for infection. To do so, pick up 10 µL of bacterial suspension and proceed with serial dilutions and spread each dilution on GC agar plates and incubate at 37 °C with 5% CO2, for 18-24 h.
- Intra-cisternal injection of mice
NOTE: The entire procedure is performed in the laminar flow cabinet to maintain aseptic conditions.- House laboratory mice (8 week-old, female Balb/c) under specific pathogen free conditions. Provide food pellets and water ad libitum.
- Settle the animals in the new environment for 1 week before starting the experiment.
- Before starting the experiment, weigh and evaluate their body temperature.
NOTE: The inbred Balb/c female mice of eight-week-old weigh an average of about 19 g. The average temperature of laboratory mice physiologically ranging from 36-38 °C24. - Scruff the animal from the neck, clean the abdomen area with the 70% ethanol and inject i.p. iron dextran (dissolved in 1 % phosphate saline buffer, 250 mg/kg) in the lower right quadrant of the murine abdomen by using a 25 G needle 0.5 mm x 16 mm, approximately 2-3 h before the infection.
NOTE: The intraperitoneal injection was performed in the lower right quadrant of the murine abdomen to avoid damaging abdominal organs such as urinary bladder, cecum, etc. The administration of exogenous iron source, in the form of iron dextran, to animals prior to the infection favors the bacterial multiplication in the host14,18,27. - After 2-3 h, perform animal anesthesia with ketamine (50 mg/kg) and xylazine (3 mg/kg) and ophthalmic lubricant.
- Check for the depth of anesthesia by ensuring the absence of pain response upon pinching the toe.
- Position the mouse in sternal decubitus and carefully stretch the limbs and the cervical spine to keep the vertebral column in a straight position.
- Gently mix the bacterial suspension to maintain a consistent suspension before loading a syringe of 30 G needle x 8 mm.
NOTE: Prepare the bacterial suspension as close as possible to the injection time; meanwhile, store it at room temperature. - Based on the post thawing bacterial titer (CFU/mL), proceed with the calculation of the total CFU to be used, with respect to the total number of animals to be infected (CFU bacterial dose per number of animals). Proceed with the calculation of the exact volume to be taken from the vial to obtain the total CFU applying a proportion between the post thawing bacterial titer (CFU/mL) and the total CFU useful for the infection of n mice (post thawing bacterial titer CFU: ml = total CFU : x). Establish the final volume with respect to the total number of animals to be infected.
NOTE: In these experiments, a wide range of titer from 104 to 109 per animal was used. - Clean the surgical area with 70% Ethanol.
- Identify the injection point with the help of a needle and inject the established CFU of meningococci (wild type strain and isogenic mutant strain), or GC broth supplemented with iron dextran (5 mg/kg) as control, in a total volume of 10 µL into the cisterna magna of mice through an occipital burr hole by using a 30 G needle x 8 mm.
- Perform the cisternal inoculum by placing the needle at the craniocervical junction, specifically in the dorsal subarachnoid space. Ventroflex the head to make this space accessible28.
- Briefly, place the animal in lateral recumbency, hold the ears out of the way and flex the neck moderately (90 to 100°). Ensure that the midline of the neck and the head (from the nose to the occiput) are in perfectly parallel position to the tabletop.
- Touch the atlas wings and make sure that they overlap, eliminating axial rotation. A natural indentation can usually be touched on midline where the needle is most likely to enter the occipital hole.
- Discard the syringe and needle safely after the injection of mice with Neisseria.
- Place the animal in the cage and wait for the awakening and full recovery of movement.
- Keep the cages with infected mice under a laminar flow cabinet.
- Monitor mice, 24 h post infection, for clinical signs of coma according to the coma scale29. Coma scale: 1 = coma, 2 = does not stand upright after being turned on the back, 3 = stands upright within 30 s, 4 = stands upright within 5 s, minimal ambulatory activities, 5 = normal.
NOTE: When in animals was recorded pain, analgesia with meloxicam (5 mg/kg i.p. for duration of study) was administered. - Proceed for the animal survival or CFU counts assay on the infected animals as detailed in step 2.
- Perform euthanasia of mice with a score of 2 by cervical dislocation and record as dead for statistical analysis.
2. Animal Survival and CFU Counts
- Animal survival
- Prepare bacterial inocula at different doses (ranging from 104 to 109 CFU per mouse) to infect animals by the i.cist. route (see step 1.2).
- Inoculate control mice with GC broth, supplemented with iron dextran (5 mg/kg), in the same manner.
- Monitor animals for clinical symptoms: ruffled fur, hunched appearance, hypothermia, weight loss, lethargy, or moribund24,25,26,30, every day throughout the whole experiment for 168 h (7 days) at least twice a day for the duration of the experiment.
- Measure the body weight and temperature by using a digital balance and a rectal thermometer, respectively everyday.
- Record the survival of mice for a week.
NOTE: Record the natural death of animal post infection while the animals that reach a coma value of 2 or that survive over 168 h of observation will be euthanized. - Anesthetize mice with a coma value of 2 or that survive over the observation time with ketamine (50 mg/kg) and xylazine (3 mg/kg) and apply ophthalmic ointment.
- Check that the pain response is absent by toe pinch.
- Perform euthanasia of mice by cervical dislocation and record as dead for statistical analysis.
- Evaluation of colony forming units (CFU) counts in peripheral organs
- Use a sub-lethal bacterial dose (5 x 105CFU/mice) on the basis of animal survival results to inoculate animals by the i.cist. route (see subsection 1.2).
- Monitor the rectal temperature every day during the infection and perform anesthesia of animals at 48 h post infection as mentioned in step 2.
- Proceed with the 70% ethanol disinfection of the chest and withdraw 600-700 µL of blood by cardiac puncture of the chest cavity using a 25 G needle 0.5 mm x 16 mm.
- Collect the blood in a tube containing 3.8% sodium citrate and store at -80 °C for the later viable bacterial cell counts.
- Perform cervical dislocation to sacrifice the animals. Confirm the death recording the absence of heartbeat, after sacrificing the mouse according to all relevant institutional and ethical guidelines.
- Lay the mouse in the supine position and use scissors and forceps to proceed with the cut of the fur along the sagittal plane of body. Fix the skin with the fur on the sides of the body with pins.
- Cut off the peritoneal membrane using sharp scissors. Use scissors and disposable forceps to excise the organs (e.g., spleen and liver) and put each one in sterile Petri dish with 1 mL of GC broth supplemented with 10% (vol/vol) glycerol.
- Safely dispose the mouse body as per IACUC guidelines.
- Homogenize the organs mechanically at room temperature with the plunger of a 5 mL syringe for about 2-3 min until a single-cell suspension is formed and transfer it in a tube.
- Put the tube with the homogenized tissue sample immediately on the dry ice.
NOTE: Samples can be stored at -80 °C in a 2 mL sterile tube for performing the viable bacterial cell counts evaluation at later points. - Make GC agar plates with antibiotics when required. Dry the plates prior to the use pre-incubating at 37 °C for 2-3 h.
- Prepare 10-fold serial dilutions of the samples in GC broth from each homogenized tissue and plate onto GC agar plates. Incubate overnight at 37 °C with 5% CO2.
3. Preparation of Brain Tissues for CFU Count
- Use a sub-lethal bacterial dose (5 x 105 CFU/mice) on the basis of animal survival results to inoculate animals by the i.cist. route (see subsection 1.2).
- Perform anesthesia of animals at established infection time as mentioned in step 2.
- Perform euthanasia of animals by cervical dislocation.
- Clean the surgical area with 70% Ethanol.
- Cut off the head of mouse using a large scissors.
- Resect the fur and the skin with the help of small surgical scissors and a fine tipped steel forceps, proceeding towards the top of the skull to be able to clearly see the sutures and to guide the opening of the cranium.
- Insert the tip of a tiny scissors through the foramen magnum to open the skull.
- Cut towards the center of the cranium and across the midline of parietal bone to the opposite side of the skull. Gently cut along the lateral rim of the lambdoid suture.
- Lift the cranium starting from the posterior parietal corner and pull it diagonally upward to discover the brain, by using fine tipped forceps.
- Be sure that the brain tissue is not attached to any bone of the head, when the skull is lifted.
- Use disposable forceps to remove any connective tissue between the skull and the brain, to ensure that brain tissue being removed along with the skull.
- Do not allow the brain tissue dry out too much. Place the brain, using disposable forceps, in a Petri dish with 1 mL of GC broth supplemented with 10% (vol/vol) glycerol.
- Homogenize the brain mechanically with the plunger of a 5 mL syringe (see step 2.2.9). Transfer the samples into a 2 mL sterile tube and store -80 °C for the later viable bacterial cell counts evaluation as discussed in step 2.2.
Representative Results
Survival of mice infected with N. meningitidis wild type and isogenic mutant strains.
The Neisseria meningitidis strains used in these representative results are the serogroup C reference strain 93/4286 (ET-37) and its isogenic mutant 93/4286ΩcssA obtained by insertional inactivation of the cssA gene, coding for the UDP-N-acetylglucosamine 2-epimerase, that maps in capsule synthesis locus25. To assess the virulence degree of the cssA-defective strain in the present murine model, the lethal dose able to determine the death of 50% of infected animals (LD50) was evaluated. To this purpose three groups of animals were infected intracisternally with doses ranging from 104 to 106 CFU of the wild type strain 93/4286 and with doses of mutant strain 93/4286ΩcssA between 107 to 109 CFU. Generally, the reduction of clinical parameters (e.g., body weight and temperature) and the increasing of mortality rate happened within the first 72 h after the infection. The LD50 for the wild type strain corresponded to the meningococcal challenge of 104 CFU, whereas the mortality rate with the dose of 105 CFU was equal to 83.4% and with 106 CFU of 100% (Figure 1A). Conversely, to obtain the LD50 for the mutant strain 93/4286ΩcssA, it has been necessary a dose of 108 CFU (Figure 1B), an amount of 10,000 folds higher compared to wild type strain.
Evaluation of N. meningitidis viable CFU in the mouse brain tissues.
To follow the kinetics of infection in the brain tissue of infected animals, a time course assay was performed with wild type or cssA mutant strain25. After the i.cist. injection with 5×105 CFU of 93/4286 or 93/4286ΩcssA strains, there was a rapid increase of wild type bacteria in the brain tissue reaching the highest numbers at around 24 h post infection (Figure 2A); conversely in the brain of mice challenged with the isogenic cssA-defective mutant, the viable counts dropped progressively over time until to 2.026 log CFU ± 1.774 72 h post infection (Figure 2A). The experiment has shown that 33.3% of the mutant challenged mice showed bacterial clearance from the infection site, whereas infection with wild type strain was never eradicated from the brain of animals.
Evaluation of meningococcal load in spleen and liver 48h post-challenge.
This experiment was performed to evaluate the clearance of bacteria from infected mice 48 h post-challenge in peripheral organs. To this aim, two groups of mice were infected with 5 x 105 CFU of either 93/4286 or 93/4286ΩcssA strains, and bacterial viable counts were evaluated in the spleen and liver of infected mice25 (Figure 2B). Following 48 h from the meningococcal injection, the cssA-defective mutant was completely cleared in spleen and liver, while the animals infected with wild type strain exhibited a persistent systemic infection framework. The mean values of CFU after 48 h were indeed still 3.212 log CFU ± 3.354 and 6.949 log CFU ± 1.37 in the spleen and liver, respectively. The difference in bacterial loads in the liver tissue of two animal groups was statistically significant (with a P < 0.001).
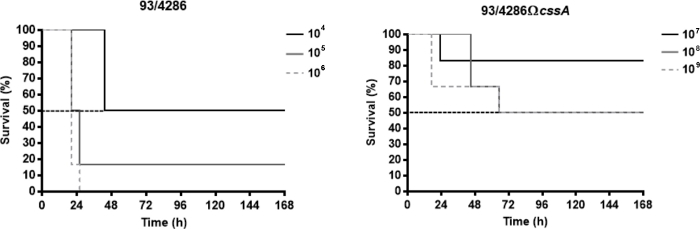
Figure 1: Survival of mice infected with 93/4286 wild type or cssA-defective N. meningitidis strains. (A) Three groups of Balb/c mice (n= 6/dose) were infected i.cist. with 104, 105, and 106 CFU/mouse of the wild type strain 93/4286 and (B) with 107, 108, and 109 CFU/mouse of the isogenic cssA-defective mutant. Mice were monitored for a week, and survival was recorded. Results are expressed as percent survival at different doses over time, the log rank p value was < 0.05 for mice infected with the wild type strain. This figure has been modified from Colicchio et al.25. Please click here to view a larger version of this figure.
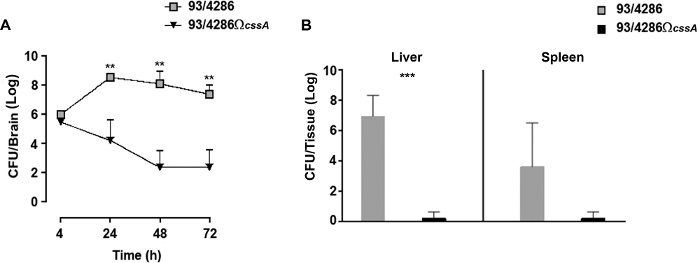
Figure 2: Evaluation of bacterial loads over time in mice inoculated with the 93/4286 or 93/4286ΩcssA strains. (A) Time course of bacterial loads in the brain tissues following i.cist. infection. Two groups of Balb/c mice (n = 20/group) were infected by the i.cist. route with 5 x 105 CFU of either the wild type strain 93/4286 or the cssA-defective mutant. Animals were sacrificed 4, 24, 48, and 72 h after infection. Brains were harvested, mechanically homogenized in GC medium, and viable counts were determined. Results are expressed as mean ±SD log of CFU numbers per organ at different time points after inoculation. Asterisks indicate statistical significance (**, P < 0.01). (B) Bacterial loads over time in spleen and liver. Two groups of Balb/c mice (n = 5/group) were infected i.cist. with 5 x 105 CFU of either the wild type strain 93/4286 or the cssA-defective mutant. Animals were euthanized 48 h after infection. Spleens and livers were harvested, mechanically homogenized, and viable counts were determined. Results are expressed as log CFU numbers per organ. Horizontal bars indicate mean logs of bacterial titers. Asterisks indicate statistical significance (***, P < 0.001). This figure has been modified from Colicchio et al.25. Please click here to view a larger version of this figure.
Discussion
In this study, we describe an experimental protocol to induce meningococcal meningitis in adult mice by i.cist. inoculation of meningococcal bacteria. To our knowledge, no other model of meningococcal meningitis has been developed in laboratory mice infected by i.cist. route; in the past, this way has been explored to provide models of meningococcal meningitis in both rat31 and rabbit32. It is well-known that the highest rate of meningococcal disease is found between young children, adolescents, and young adults33,34,35; for this reason, in our meningitis mouse model, instead of focusing on neonatal or infant animals, 8 week-old immunocompetent animals were employed.
In our experimental model, we decided to use i.cist. inoculum as it ensures the releasing of the meningococci directly into cisterna magna so facilitating bacterial replication in the CSF. This route of inoculation is physiologically more accessible28 and less traumatic than the intracranial subarachnoidal route, already used for the development of meningitis due to Streptococcus spp.36,37. Although it does not represent the natural way of infection of meningococcus, the injection of bacteria in this area was instrumental for the induction of meningococcal meningitis, as shown by mouse survival, bacteria loads, clinical parameters, and also by histological analysis24,25,26. Interestingly, reference strain 93/4286 induced meningitis with histopathologic features mimicking those observed in human disease24,25,26.
To establish a standardized murine infection and to guarantee the safety of researchers, it was preferred to start from a titrated frozen stock of bacteria rather than a fresh bacterial growth24,25,30,38, moreover, we decided to use a sub-lethal dose of live meningococci with the aim to limit a rapid fatal outcome and permit the development of brain damage2,24,25,26. Nevertheless, to determine an appropriate infectious dose for different strains, preliminary experiments must be performed. In the present study, we tested a serogroup C reference strain 93/4286 and an isogenic mutant strain 93/4286ΩcssA with a dose of 5 x 105 CFU/ mice.
Although this model does not mimic the initial phases of colonization and invasion of meningococcus, the bacterial isolate grows well not only in the CSF, but it is also able to remain in the spleen and liver compartments. During the hypoferremic phase of neisserial infection, most of heme-derived iron remains combined with liver ferritin. As ferritin can be used meningococci to obtain iron39, the liver constitutes a target organ for bacterial replication.
In this experimental procedure, the inbred Balb/c mouse strain was used in replacement of the outbred CD-1 strain that was originally employed to develop the meningococcal meningitis model24. Outbred mice were characterized by a wide genetic variability that may be more appropriate to reveal several effects in a variable cohort such as human population40,41. However, this variability needs a higher sample size to obtain sufficient statistical significance and may interfere with the standardization of procedures and targeted studies.
Despite the narrow host range of meningococcus, to ensure the replication of bacteria in murine host and enhance the virulence of meningococci, iron dextran was administered to animals before the infection6,14. Finally, compared to other experimental model of meningitis, animals were not treated with any antibiotics, to not affect the course of disease and/or profile of the inflammatory response26.
To date, our studies have highlighted that the i.cist. model is functional to induce both meningitis and invasive meningococcal disease compared to i.p. or i.n. models of infection, which are characterized by the occurrence of sepsis and bacteremia before meningitis is established10,11,12,13,14,15,16,17. Therefore, this infection model based on the induction of meningitis in murine host, could be useful not only to evaluate innovative therapeutic strategy to prevent bacterial replication directly into CSF but also to analyze the efficacy of possible passive immune therapy against human pathogens.
However meningococcal infection is a multistep process, that includes nasopharynx colonization, access to bloodstream, crossing of the blood brain barrier and finally uncontrolled proliferation in the CSF, our model only reproduces some aspects of the meningococcal infection, in order to subvert in part these limitations transgenic animal model may be useful to mimic the human pathogenesis of meningococcal disease.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The studies were supported in part by PRIN 2012 [grant number 2012WJSX8K]: “Host-microbe interaction models in mucosal infections: development of novel therapeutic strategies” and by PRIN 2017 [2017SFBFER]: “An integrated approach to tackle the interplay among adaptation, stressful conditions and antimicrobial resistance of challenging pathogens”.
Materials
| 1,8 Skirted Cryovial With external thread | Starlab | E3090-6222 | |
| 50ml Polypropylene Conical Tube | Falcon | 352070 | 30 x 115mm |
| Adson Forceps | F.S.T. | 11006-12 | Stainless Steel |
| Alarm-Thermometer | TESTO | 9000530 | |
| BactoTM Proteose Peptone | BD | 211693 | |
| BD Micro Fine syringe | BD | 320837 | U-100 Insulin |
| BD Plastipak syringe 1ml 25GA 5/8in | BD | 300014 | 05x16mm |
| BD Plastipak syringe 5ml | BD | 308062 | 07 x 30mm |
| BIOHAZARD AURA B VERTICAL LAMINAR FLOW CABINET | Bio Air s.c.r.l. | Aura B3 | |
| BioPhotometer | Eppendorf | Model #6131 | |
| Bottle D | Tecniplast | D | Graduated up to:400ml, Total Volume 450ml, 72x72x122mm |
| C150 CO2 Incubator | Binder | 9040-0078 | |
| Cage Body Eurostandard Type II | Tecniplast | 1264C | 267x207x140mm, Floor area 370cm2 |
| Cell Culture Petri Dish With Lid | Thermo Scientific | 150288 | Working Volume: 5mL |
| Centrifuge | Eppendorf | Microcentrifuge 5415R | |
| Cuvetta semi-micro L. Form | Kartell S.p.A. | 01938-00 | |
| di-Potassium hydrogen phosphate trihydrate | Carlo erba | 471767 | |
| di-Sodium hydrogen phosphate anhydrous ACS-for analysis | Carlo Erba | 480141 | g1000 |
| Diete Standard Certificate | Mucedola s.r.l. | 4RF21 | Food pellet for animal |
| Dumont Hp Tweezers 5 Stainless Steel | F.S.T. by DUMONT | AGT5034 | 0,10 x 0,06 mm tip |
| Electronic Balance | Gibertini | EU-C1200 | Max 1200g, d=0,01g, T=-1200g |
| Eppendorf Microcentrifuge tube safe-lock | Eppendorf | T3545-1000EA | |
| Erythromycin | Sigma-Aldrich | E-6376 | 25g |
| Extra Fine Bonn Scissors | F.S.T. | 14084-08 | Stainless Steel |
| Filter Top (mini- Isolator), H-Temp with lock clamps | Tecniplast | 1264C400SUC | |
| GC agar base | OXOID | CM0367 | |
| Gillies Forceps 1 x2 teeth | F.S.T. | 11028-15 | Stainless Steel |
| Glicerin RPE | Carlo Erba | 453752 | 1L |
| Graefe Forceps | F.S.T. | 11052-10 | Serrated Tip Width: 0.8mm |
| Inner lid | Tecniplast | 1264C116 | |
| Iron dextran solution | Sigma-Aldrich | D8517-25ML | |
| Ketamine | Intervet | ||
| Microbiological Safety Cabinet BH-EN and BHG Class II | Faster | BH-EN 2004 | |
| Microcentrifuge tubes 1.5ml | BRAND | PP780751 | screw cap PP, grad |
| Mouse Handling Forceps | F.S.T. | 11035-20 | Serrated rubber; Gripping surface:15 x 20 mm |
| Mucotit-F2000 | MERZ | 61846 | 2000ml |
| Natural Latex Gloves | Medica | M101 | |
| New Brunswick Classic C24 Incubator Shaker | PBI international | C-24 Classic Benchtop Incubator Shaker | |
| Petri PS Dishes | VWR | 391-0453 | 90X14.2MM |
| Pipetman Classic P20 | Gilson | F123600 | 2-20microL |
| Pipetman Classic P200 | Gilson | F123601 | 20-200microL |
| Pipetman Classim P1000 | Gilson | F123602 | 200-1000microL |
| Polyvitox | OXOID | SR0090A | |
| Potassium Chloride | J.T. Baker Chemicals B.V. | 0208 | 250g |
| Potassium Dihydrogen Phosphate | J.T. Baker Chemicals B.V. | 0240 | 1Kg |
| PS Disposible forceps | VWR | 232-0191 | |
| Removable Divider | Tecniplast | 1264C812 | |
| Round-Bottom Polypropylene Tubes | Falcon | 352063 | 5ml |
| Sodium Chloride | MOLEKULA | 41272436 | |
| SS retainer and Polyester FilterSheet | Tecniplast | 1264C | |
| Standard Pattern Forceps | F.S.T. | 11000-12 | Stainless |
| Stevens Tenotomy Scissors | F.S.T. | 14066-11 | Stainless Steel |
| Surgical Scissor – ToughCut | F.S.T. | 14130-17 | Stainless |
| Touch N Tuff disposible nitrile gloves | Ansell | 92-500 | |
| Ultra Low Temperature (ULT) Freezer | Haier | DW-86L288 | Volume= 288L |
| Wagner Scissors | F.S.T. | 14070-12 | Stainless Steel |
| Xylazine | Intervet |
References
- van Deuren, M., Brandtzaeg, P., van der Meer, J. W. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clinical Microbiology Reviews. 13, 144-166 (2000).
- Colicchio, R., et al. Fitness Cost of Rifampin Resistance in Neisseria meningitidis: In vitro Study of Mechanisms Associated with rpoB H553Y Mutation. Antimicrobial Agents and Chemotherapy. 59 (12), 7637-7649 (2015).
- Talà, A., et al. Serogroup-specific interaction of Neisseria meningitidis capsular polysaccharide with host cell microtubules and effects on tubulin polymerization. Infection and Immunity. 82, 265-274 (2014).
- Pagliarulo, C., et al. Regulation and differential expression of gdhA encoding NADP-specific glutamate dehydrogenase in Neisseria meningitidis clinical isolates. Molecular Microbiology. 51, 1757-1772 (2004).
- Plant, L., Jonsson, A. B. Contacting the host: insights and implications of pathogenic Neisseria cell interactions. Scandinavian Journal of Infectious Diseases. 35, 608-613 (2003).
- Schryvers, A. B., Stojiljkovic, I. Iron acquisition systems in the pathogenic Neisseria. Molecular Microbiology. 32, 1117-1123 (1999).
- Virji, M., Makepeace, K., Ferguson, D. J., Watt, S. M. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Molecular Microbiology. 22, 941-950 (1996).
- de Vries, F. P., van Der Ende, A., van Putten, J. P., Dankert, J. Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infection and Immunity. 64, 2998-3006 (1996).
- Tinsley, C. R., Heckels, J. E. Variation in the expression of pili and outer membrane protein by Neisseria meningitidis during the course of the meningococcal infection. Journal of General Microbiology. 132, 2483-2490 (1986).
- Gorringe, A. R., et al. Experimental disease models for the assessment of meningococcal vaccines. Vaccine. 23, 2214-2217 (2005).
- Newcombe, J., et al. Infection with an avirulent phoP mutant of Neisseria meningitidis confers broad cross-reactive immunity. Infection and Immunity. 72, 338-344 (2004).
- Oftung, F., Lovik, M., Andersen, S. R., Froholm, L. O., Bjune, G. A mouse model utilising human transferrin to study protection against Neisseria meningitidis serogroup B induced by outer membrane vesicle vaccination. FEMS Immunology and Medical Microbiology. 26, 75-82 (1999).
- Salit, I. E., Tomalty, L. Experimental meningococcal infection in neonatal mice: differences in virulence between strains isolated from human cases and carriers. Canadian Journal of Microbiology. 30, 1042-1045 (1984).
- Salit, I. E., Tomalty, L. A neonatal mouse model of meningococcal disease. Clinical and Investigative Medicine. 9, 119-123 (1986).
- Mackinnon, F. G., Gorringe, A. R., Funnell, S. G., Robinson, A. Intranasal infection of infant mice with Neisseria meningitidis. Microbial Pathogenesis. 12, 415-420 (1992).
- Mackinnon, F. G., et al. Demonstration of lipooligosaccharide immunotype and cap- sule as virulence factors for Neisseria meningitidis using an infant mouse intranasal infection model. Microbial Pathogenesis. 15, 359-366 (1993).
- Yi, K., Stephens, D. S., Stojiljkovic, I. Development and evaluation of an improved mouse model of meningococcal colonization. Infection and Immunity. 71 (4), 1849-1855 (2003).
- Holbein, B. E., Jericho, K. W. F., Likes, G. C. Neisseria meningitidis infection in mice: influence of iron, variations in virulence among strains, and pathology. Infection and Immunity. 24, 545-551 (1979).
- Saukkonen, K. Experimental meningococcal meningitis in the infant rat. Microbial Pathogenesis. 4, 203-211 (1988).
- Johansson, L., et al. CD46 in meningococcal disease. Science. 301, 373-375 (2003).
- Zarantonelli, M. L., et al. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infection and Immunity. 75, 5609-5614 (2007).
- Join-Lambert, O., et al. Meningococcal interaction to microvasculature triggers the tissular lesions of purpura fulminans. Journal of Infection Disease. 208, 1590-1597 (2013).
- Melican, K., Michea Veloso, P., Martin, T., Bruneval, P., Duménil, G. Adhesion of Neisseria meningitidis to dermal vessels leads to local vascular damage and purpura in a humanized mouse model. PLoS Pathogen. 9, 1003139 (2013).
- Colicchio, R., et al. The meningococcal ABC-Type L-glutamate transporter GltT is necessary for the development of experimental meningitis in mice. Infection and Immunity. 77, 3578-3587 (2009).
- Colicchio, R., et al. Virulence traits of serogroup C meningococcus and isogenic cssA mutant, defective in surface-exposed sialic acid, in a murine model of meningitis. Infection and Immunity. , 00688-00718 (2019).
- Ricci, S., et al. Inhibition of matrix metalloproteinases attenuates brain damage in experimental meningococcal meningitis. BMC Infectious Diseases. 14, 726 (2014).
- Schryvers, A. B., Gonzalez, G. C. Comparison of the abilities of different protein sources of iron to enhance Neisseria meningitidis infection in mice. Infection and Immunity. 57, 2425-2429 (1989).
- Beverly, K. S. Chapter 105 – Cerebrospinal Fluid Sampling Small Animal. Critical Care Medicine. , 448-452 (2009).
- Liechti, F. D., Grandgirard, D., Leppert, D., Leib, S. L. Matrix metalloproteinase inhibition lowers mortality and brain injury in experimental pneumococcal meningitis. Infection and Immunity. 82, 1710-1718 (2014).
- Pagliuca, C., et al. Novel Approach for Evaluation of Bacteroides fragilis Protective Role against Bartonella henselae Liver Damage in Immunocompromised Murine Model. Frontiers in Microbiology. 7, 1750 (2016).
- Trampuz, A., Steinhuber, A., Wittwer, M., Leib, S. L. Rapid diagnosis of experimental meningitis by bacterial heat production in cerebrospi- nal fluid. BMC Infectious Diseases. 7, 116 (2007).
- Tuomanen, E. I., Saukkonen, K., Sande, S., Cioffe, C., Wright, S. D. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. Journal of Experimental Medicine. 170, 959-969 (1989).
- Goldschneider, I., Gotschlich, E. C., Artenstein, M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. Journal of Experimental Medicine. 129, 1307-1326 (1969).
- Goldschneider, I., Gotschlich, E. C., Artenstein, M. S. Human immunity to the meningococcus. II. Development of natural immunity. Journal of Experimental Medicine. 129, 1327-1348 (1969).
- World Health Organization. Laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae: WHO manual, 2nd Edition. World Health Organization. , (2011).
- Chiavolini, D., et al. Method for inducing experimental pneumococcal meningitis in outbred mice. BMC Microbiolology. 4, 36 (2004).
- Zhang, S., et al. Intracranial Subarachnoidal Route of Infection for Investigating Roles of Streptococcus suis Biofilms in Meningitis in a Mouse Infection Model. Journal of Visualized Experiments. (1), e137 (2018).
- Pagliuca, C., et al. Evidence of Bacteroides fragilis protection from Bartonella henselae-induced damage. PLoS One. 7, 49653 (2012).
- Larson, J. A., Howie, H. L., So, M. Neisseria meningitidis accelerates ferritin degradation in host epithelial cells to yield an essential iron source. Molecular Microbiology. 53, 807-820 (2004).
- Festing, M. F. W. Phenotypic variability of inbred and outbred mice. Nature. 263, 230-232 (1976).
- Festing, M. F. W. Warning: the use of heterogeneous mice may seriously damage your research. Neurobiology of Aging. 20, 237-244 (1999).

