Induction and Evaluation of Inbreeding Crosses Using the Ant, Vollenhovia Emeryi
Summary
In this protocol, methods for conducting inbreeding crosses, and for evaluating the success of those crosses, are described for the ant Vollenhovia emeryi. These protocols are important for experiments aimed at understanding the genetic basis of sex determination systems in Hymenoptera.
Abstract
The genetic and molecular components of the sex-determination cascade have been extensively studied in the honeybee, Apis mellifera, a hymenopteran model organism. However, little is known about the sex-determination mechanisms found in other non-model hymenopteran taxa, such as ants. Because of the complex nature of the life cycles that have evolved in hymenopteran species, it is difficult to maintain and conduct experimental crosses between these organisms in the laboratory. Here, we describe the methods for conducting inbreeding crosses and for evaluating the success of those crosses in ant Vollenhovia emeryi. Inducing inbreeding in the laboratory using V. emeryi, is relatively simple because of the unique biology of the species. Specifically, this species produces androgenetic males, and female reproductives exhibit wing polymorphism, which simplifies identification of the phenotypes in genetic crosses. In addition, evaluating the success of inbreeding is straightforward as males can be produced continuously by inbreeding crosses, while normal males only appear during a well-defined reproductive season in the field. Our protocol allow for using V. emeryi as a model to investigate the genetic and molecular basis of the sex determination system in ant species.
Introduction
Eusocial Hymenopteran taxa, such as ants and bees, have evolved a haplodiploid sex-determination system in which individuals that are heterozygous at one or more complementary sex determination (CSD) loci become females, while those that are homo- or hemizygous become males (Figure 1A)1.
Genetic and molecular components involved in the sex determination cascade have been well studied in the honeybee, Apis mellifera, a hymenopteran model organism2,3,4. Recent comparative genomics investigations suggest that ants and honeybees share many putative homologs in the sex determination pathway, such as the initial sex determination gene, csd5. However, evidence for the functional conservation of these homologs is still lacking in ants.
To address this problem, inbreeding lines need to be developed as they are essential for genetic mapping and molecular studies. However, it is difficult to maintain and conduct experimental crosses between these organisms in the laboratory because of the complex nature of the life cycles that have evolved.
Here, we use Vollenhovia emeryi as a model to investigate the genetic and molecular basis of the sex determination system in ants6,7. The inbreeding lines of this species were developed previously for linkage mapping of quantitative trait loci (QTL) for traits related to sex determination for the first time in ants6. In addition, the molecular sex-determination cascade has been investigated7. This species has evolved an unusual reproduction system that employs both gynogenesis and androgenesis (Figure 1B)8,9. Most new queens and males are clonally produced from the maternal and paternal genomes, respectively. In addition, workers and some queens are produced sexually8. This reproduction system is particularly well suited to genetic studies because the inbreeding crosses produced using sexually produced queens and males are genetically equivalent to a classic backcross. Since sexually produced queens differ morphologically from queens produced from maternal genomes10 (Figure 1B), conducting and evaluating inbreeding crosses is greatly simplified using this method.
In this article, the methods for establishment of laboratory colonies for crossing test, application of inbreeding crosses using full-sib pairs, and evaluating the success of those crosses using genotyping of colony members and dissection of male offspring genitalia are described in V. emeryi.
Regardless of the reproduction system employed, application of inbreeding crosses is often the essential first step in any investigation of sex determination systems in the Hymenoptera. For example in Cardiocondyla obscurior, the almost complete absence of diploid males after 10 generations of full-sib mating in the laboratory demonstrates absence of CSD locus11. It is possible to predict the number of CSD loci from the ratio of males produced in inbreeding crosses6,12,13.
Protocol
1. Field Collection and Maintenance of V. Emeryi Colonies in the Laboratory
NOTE: Nests of V. emeryi are found in rotting logs and fallen decaying tree branchesin secondary forests throughout Japan. This species shows two types of colonies, i.e., (1) colonies producing only long-winged queens and (2) colonies mainly producing short-winged queens in addition to small number of long-winged queens8,14. In this protocol, we collected the latter type of colonies in Ishikawa prefecture, Japan.
- Collect V. emeryi colonies during early summer.
NOTE: To obtain sufficient numbers of sexual individuals during the reproductive season, colonies containing more than 300 individuals are preferred. - Transfer the ant specimens from the collected branches to an artificial plaster nest with a glass cover using an aspirator (Figure 2, left).
- Maintain colonies in the artificial nest at 25 °C under a 16:8 h light/dark cycle. Provide tap water with a wash bottle to wet the plaster.
- Add about 100 mg of dry cricket powder wrapped in aluminum foil and a brown sugar water-filled tip (20 µL tip) every other day until new reproductives (F1 winged-queens and F1 males) emerge.
2. Experimental Laboratory Crosses
NOTE: New reproductives start to emerge from late summer to autumn (Figure 3). Long-winged queens are produced sexually, and short-winged queens are produced clonally and have the maternal genome (Figure 1). Use long-winged queens and males for inbreeding crosses.
- To stop individuals from moving, place colonies in a constant environment room at 4 °C for 15 min.
- Remove the mid-legs of 30 workers using forceps under a stereoscopic microscope and transfer them into new smaller plaster nest (Figure 2, right) for inbreeding crosses.
NOTE: The legs are removed to distinguish the present workers from the workers that will be produced by the subsequent inbreeding crosses. - Add 3-4 larvae or pupae into a plaster nest containing workers.
NOTE: Workers show exploratory activity in the F0 queens-less colony. Larvae or pupae can effectively attract these workers and new reproductives in the center of the colony during crossing test. As a result, keep conditions of the experimental colony close to the normal colony. - Transfer a long-winged queen and a male into a plaster nest prepared in step 2.3 for inbreeding crosses.
- Keep colonies at 25 °C under a 16:8 h light/dark cycle with food and water provided as described in 1.3 until the queen lose her wings and lay eggs.
NOTE: This takes one week to a month. - Check the experimental colony everyday under a stereoscopic microscope. After performing inbreeding crosses between the F1 offspring, eggs can be observed under a stereoscopic microscope.
- After the F1 queen starts laying eggs, remove F1 males and larvae or pupae added in step 2.3 from the nest to avoid mixing of the F1 generation (males and females used for inbreeding crosses) and the F2 generation (offspring produced from inbreeding crosses).
NOTE: If there are few males in the colony, it is possible to induce inbreeding crosses using one male and 1 to 3 queens in the same experimental colony. - Keep colonies under the same conditionsas described in 1.3, until F2 offspring emerge.
NOTE: Transfer F1 queen and F2 offspring into new larger plaster nest (Figure 2, left) for long-term colony keeping.
3. Evaluation of Inbreeding Success
- DNA extraction and genotyping of the parental generation (F0)
- Remove one leg of a F0 queen using forceps and transfer the leg to a 1.5 mL microtube containing 100 µL of chelation agent.
- Under a stereoscopic microscope, dissect a female abdomen in glass dish filled with 300 µL of ultrapure water using forceps and isolate the spermatheca containing the sperm from mated males.
- Peel away the tissue of the spermatheca and isolate the sperm from the tissue of the female using insect pins.
NOTE: To facilitate sperm extraction from the spermatheca, store female specimens in 100% EtOH for more than one day before dissection. - Using a micropipette, transfer the sperm into a 1.5 mL microtube containing 100 µL of chelation agent.
- Incubate samples of F0 queen and sperms prepared in step 3.1.1 and 3.1.3, respectively, at 95 °C for 20 min. Flash centrifuge the microtube and store at 4 °C.
- Genotype all samples using method described elsewhere4.
- DNA extraction from the pair of ants used for inbreeding crosses (F1)
- After confirming egg production by the sib mated F1 queen, extract the DNA of the queen using her shed wings or one mid-leg and genotype using same method described in section 3.1 above.
- Extract DNA of F1 male using one leg and genotype them using same method described in section 3.1 above.
NOTE: Samples can be stored in 100% EtOH before DNA extraction, and DNA in chelation agent can be stored for two months at 4 °C.
- Evaluation of male fertility in males produced from inbreeding crosses
NOTE: Diploid males produced from inbreeding crosses are often sterile.- Dissect internal reproductive organs in a glass dish with 400 µL of PBS solution using forceps.
- Remove PBS and add 4% paraformaldehyde (PFA) using a micropipette.
- Fix the tissue by incubating in PFA for 30 min at room temperature (15 – 25 °C).
- Wash tissue 5 times with 400 µL of PBS using a micropipette.
- Dilute the 4',6-diamidino-2-phenylindole (DAPI) solution to 1 µg/mL in PBS.
- Remove PBS and add approximately 300 µL of this dilute DAPI staining solution to tissue.
- Incubate 15 min under dark condition at room temperature (15 – 25 °C).
- Wash tissue 5 times with 400 µL of PBS, and transfer tissue on center of slide glass using forceps.
- Mount tissue on mounting medium containing Tetramethylrhodamine (TRITC)-conjugated phalloidin.
- Observe samples by confocal laser scanning microscope using 20X or 63X objective lenses.
- Use a 405 nm excitation laser and a hybrid detector at 410-530 nm for DAPI detection.
- Use a 561 nm excitation laser and a hybrid detector at 565-650 nm for TRITC detection.
- Use a scan speed of 400 Hz (400 lines/s) at a resolution at 1024 × 1024 pixels.
- Capture images using a software platform.
Representative Results
Results of microsatellite analysis using F0 and F1 generations showed that inbreeding crosses were produced successfully (Figure 4)6. As a result of inbreeding crosses, mated queens were obtained within one month of establishing the experimental crossing colonies. A quarter (27.1 ± 8.91% SD) of all offspring (F2) from the inbreeding crosses was male, while the remainder was female (workers and a queen)6. QTL mapping using the offspring from inbreeding crosses showed that males produced by inbreeding crosses were diploid and homozygous at two CSD loci (CSD1 and CSD2 in Figure 5), while females (workers) produced from inbreeding crosses were diploid and heterozygous at least at one CSD locus6.
Dissection of haploid males revealed testes and sperm, as expected (Figure 6A–6C). However, in diploid males, sperms were never observed, suggesting that males produced in inbreeding crosses are sterile in V. emeryi6. In addition, testes of diploid males failed to develop (Figure 6D–6E).
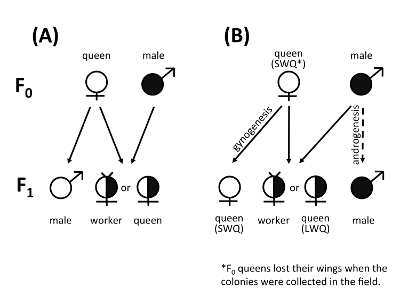
Figure 1. Typical reproductive system in (A) Hymenoptera and the atypical reproductive system involving androgenesis and gynogenesis in (B) V. emeryi. Typically, females (workers and queens) develop from fertilized diploid eggs, and males develop from unfertilized haploid eggs containing half of the maternal genome (A). In V. emeryi, sterile workers and a few long-winged queens (LWQ) develop from fertilized diploid eggs, while short-winged queens (SWQ) develop with nearly complete maternal genomes from unfertilized diploid eggs (gynogenesis). Males never inherit maternal genomes but are clones of their fathers (androgenesis) (B). This figure has been modified from [Miyakawa et al. 2018]7. Please click here to view a larger version of this figure.
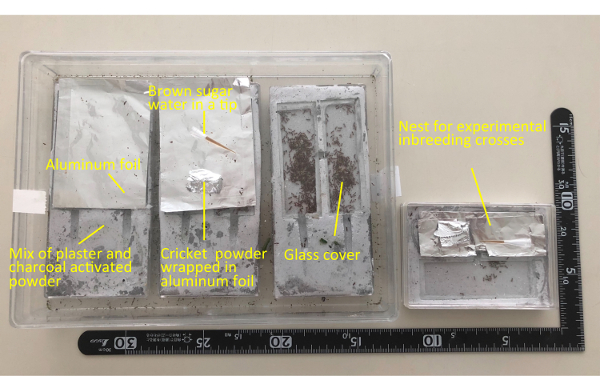
Figure 2. Experimental set up of V. emeryi colonies. After field collection, colonies are transferred into an artificial plaster nest and kept in the laboratory. A large plaster nest (left) is prepared for maintain collected colonies, whereas a smaller plaster nest (right) is prepared for experimental inbreeding crosses. Please click here to view a larger version of this figure.

Figure 3. New V. emeryi reproductives emerge during the reproductive season. Mature and well-fedcolonies tend to produce long-winged queens (LWQ) with the parental genome in addition to short-winged queens (SWQ) which bear only the maternal genome (Figure 1B). Photo courtesy of Mr. Taku Shimada. Please click here to view a larger version of this figure.
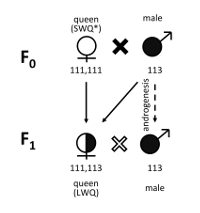
Figure 4. Design of inbreeding crosses and microsatellite genotypes of F0 and F1 generations. Using 11 microsatellite markers developed in previous studies6,8,9, females and males of the parental generation (F0) showed different genotypes. The genotypes of females and males used for experimental crosses (F1) inherited the parental and paternal genotypes, respectively, indicating that females were crossed successfully with their brothers, with which the females shared half their genomes. Numbers indicate lengths of PCR products at microsatellite locus L-5, which is one of the markers used for genotyping6,8,9,10. This figure has been illustrated according to the data from [Miyakawa and Mikheyev 2015]6. Please click here to view a larger version of this figure.
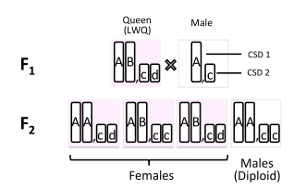
Figure 5. Allele patterns of two CSD loci (CSD1 and CSD2) in offspring produced by inbreeding crosses. Proportion of diploid males (about 25%) and QTL mapping using offspring produced by sib-mated queens suggest the existence of two CSD loci in V. emeryi. Females are heterozygous in at least one of the two CSD loci whereas males are homozygous at all loci. Genotypes are represented by letters of the alphabet. This figure has been modified from [Miyakawa et al. 2018]7. Please click here to view a larger version of this figure.
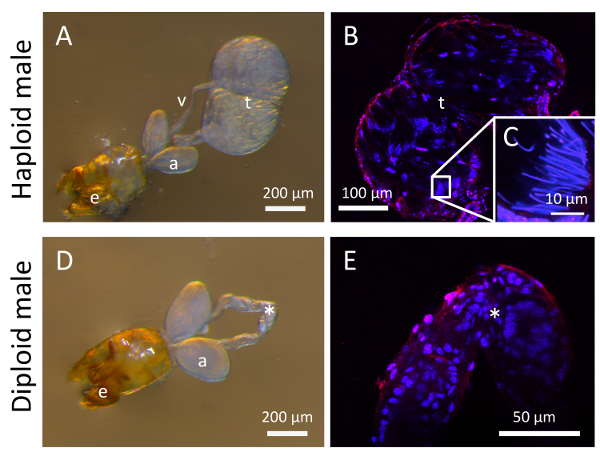
Figure 6. Male internal reproductive organs of androgenetic haploid and diploid males in V. emeryi. Morphologies of testes and other internal reproductive organs dissected out from the androgenetic haploid males (A). Sperm (fibrous tissue) could be seen in testes of haploid males (B and C). Blue color marks nuclei stained by DAPI, and red color marks F-actin stained by Tetramethylrhodamine (TRITC)-conjugated phalloidin in B, C, and E. (a) accessory glands; (t) testes; (v) vas deferens; (g) external genitalia. Morphologies of internal reproductive organs dissected out from the diploid males (D). Testes and sperm of diploid males were never observed (D and E, N >> 30). Please click here to view a larger version of this figure.
Discussion
This article demonstrates protocols that can be used to induce inbreeding crosses and evaluate the occurrence of inbreeding in the ant V. emeryi. In the experiments, genotyping of the individuals used for crosses is necessary to ensure that inbreeding crosses were successful. However, the effectiveness of these crossing tests is clearly apparent as diploid males can be produced throughout the year, while haploid males can only be produced in autumn in both the field and the laboratory6. Sib-mated queens start to produce male offspring immediately after crossing. No morphological phenotypic differences were observed between diploid and haploid males in V. emeryi6,7. However, diploid male V. emeryi almost invariably fail to develop testes. In the absence of genetic markers for testing the genetic relatedness of pairs used for crossing tests, the reproductive potential of male offspring can be used to infer whether inbreeding has occurred. However, it should be noted that males produced in inbreeding crosses are not always sterile in other hymenopteran species15,16,17.
The first critical step in the success of the protocol is the maintenance of well-fed colonies, as feeding them after field collection will increase the likelihood of obtaining sufficient numbers of reproductives for crosses. In V. emeryi, a positive correlation has been reported between nutrition and the production of long-winged queens, which are the queens used for the inbreeding crosses10. In social insects, small colonies, or colonies in poor health, tend not to produce new reproductives18. It is therefore important to collect mature colonies from the field and to provide them with adequate amounts of nutritious food for experiments using new reproductives.
The second critical step is to keep workers, reproductive and few larvae or pupae together during the crossing tests and to maintain the experimental crossing colony at the same state as that of a normal colony until crossing is completed (for a week to a month). It is difficult to maintain colonies that are to be used for crossing tests without workers for more than 3 days because males are unable to feed themselves and must be fed by workers. Under such unnatural conditions, the success rate of inbreeding crosses was extremely low6.
There are two limitations regarding the application of these protocols to other ant species. First, the cues for inducing crosses are species specific. It is relatively easy to induce laboratory crosses in V. emeryi since intra-colony mating without flight occurs in nature. However, many ant species have evolved mating rituals that involve nuptial flights during which new queens and males mate during or after flight19. It is therefore important to elucidate the triggers that induce crossing in each species in a laboratory setting. For example, in the parasitic ant Acromyrmex ameliae, the main stimulus for triggering nuptial flights appears to be light20. The second limitation is, in some cases, the diploid males produced by inbreeding crosses cannot be collected as they are inviable or killed by workers because they do not work and/or have no or low reproductive potential, and are thus a major cost to the colony of the objective species21,22,23. Fortunately, diploid V. emeryi males are not killed by workers and they live until they die naturally, which suggests that they are not frequently encountered in nature and a strategy to exclude diploid males from colonies has not evolved in this species.
Compared to other ant species that employ arrhenotokous parthenogenesis to reproduce (Figure 1A), we can assume that there are certain advantages to producing experimental inbreeding crosses using V. emeryi by androgenesis as the inbreeding crosses are genetically equivalent to a classic backcross. Indeed, the system has enabled us to design experiments to investigate the sex-determination genes, molecular mechanisms, and perform functional studies in this species6,7.
In summary, methods to conduct inbreeding crosses and to evaluate the success of the resulting crosses have been described in V. emeryi. These protocols are essential for experiments directed at understanding the genetic and molecular basis of sex determination systems in the Hymenoptera.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Mr. Taku Shimada, the delegate of AntRoom, Tokyo, Japan, for providing us with his photograph of V. emeryi reproductives. This project was funded by the Japan Society for the Promotion of Science (JSPS) Research Fellowship for Young Scientists (16J00011), and Grant in Aid for Young Scientists (B)(16K18626).
Materials
| Plaster powder | N/A | N/A | Any brand can be used |
| Charcoal, Activated, Powder | Wako | 033-02117,037-02115 | |
| Slide glass | N/A | N/A | Any brand can be used |
| Dry Cricket diet | N/A | N/A | Any brand can be used |
| Brown shuger | N/A | N/A | Any brand can be used |
| Styrene Square-Shaped Case | AS ONE | Any size | Size varies by number of ants |
| Incbator | Any brand can be used | ||
| Aluminum block bath Dry thermo unit DTU-1B | TAITEC | 0014035-000 | |
| 1.5mL Hyper Microtube,Clear, Round bottom | WATSON | 131-715CS | |
| Ethanol (99.5) | Wako | 054-07225 | |
| Stereoscopic microscope | N/A | N/A | Any brand can be used |
| Forseps | DUMONT | 0108-5-PO | |
| Chelex 100 sodium form | SIGMA | 11139-85-8 | |
| Phosphate Buffer Saline (PBS) Tablets, pH7.4 | TaKaRa | T9181 | |
| Paraformaldehyde | Wako | 162-16065 | |
| -Cellstain- DAPI solution | Dojindo Molecular Technologies | D523 | |
| VECTASHIELD Hard・Set Mounting Medium with TRITC-Phalloidin | Vector Laboratories | H-1600 | |
| ABI 3100xl Genetic Analyzer | Applied Biosystems | Directly contact the constructor formore informations. | |
| Confocal laser scanning microscope Leica TCS SP8 | Leica | Directly contact the constructor formore informations. | |
| HC PL APO CS2 20x/0.75 IMM | Leica | Directly contact the constructor formore informations. | |
| HC PL APO CS2 63x/1.20 WATER | Leica | Directly contact the constructor formore informations. | |
| Leica HyDTM | Leica | Directly contact the constructor formore informations. | |
| Leica Application Suite X (LAS X) | Leica | Directly contact the constructor formore informations. |
References
- Mable, B. K., Otto, S. P. The evolution of life cycles with haploid and diploid phases. BioEssays. 20 (6), 453-462 (1998).
- Beye, M., Hasselmann, M., Fondrk, M. K., Page, R. E., Omholt, S. W. The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell. 114 (4), 419-429 (2003).
- Hasselmann, M., et al. Evidence for the evolutionary nascence of a novel sex determination pathway in honeybees. Nature. 454 (7203), 519-522 (2008).
- Nissen, I., Müller, M., Beye, M. The Am-tra2 gene is an essential regulator of female splice regulation at two levels of the sex determination hierarchy of the honeybee. 遗传学. 192 (3), 1015-1026 (2012).
- Schmieder, S., Colinet, D., Poirié, M. Tracing back the nascence of a new sex-determination pathway to the ancestor of bees and ants. Nature Communications. 3, 895 (2012).
- Miyakawa, M. O., Mikheyev, A. S. QTL Mapping of Sex Determination Loci Supports an Ancient Pathway in Ants and Honey Bees. PLoS Genetics. 11 (11), (2015).
- Miyakawa, M. O., Tsuchida, K., Miyakawa, H. The doublesex gene integrates multi-locus complementary sex determination signals in the Japanese ant, Vollenhovia emeryi. Insect Biochemistry and Molecular Biology. 94, 42-49 (2018).
- Ohkawara, K., Nakayama, M., Satoh, A., Trindl, A., Heinze, J. Clonal reproduction and genetic caste differences in a queen-polymorphic ant, Vollenhovia emeryi. Biology letters. 2 (3), 359-363 (2006).
- Kobayashi, K., Hasegawa, E., Ohkawara, K. Clonal reproduction by males of the ant Vollenhovia emeryi (Wheeler). Entomological Science. 11 (2), 167-172 (2008).
- Okamoto, M., Kobayashi, K., Hasegawa, E., Ohkawara, K. Sexual and asexual reproduction of queens in a myrmicine ant, Vollenhovia emeryi (Hymenoptera: Formicidae). Myrmecological News. 21, 13-17 (2015).
- Schrempf, A., Aron, S., Heinze, J. Sex determination and inbreeding depression in an ant with regular sib-mating. Heredity. 97 (1), 75-80 (2006).
- De Boer, J. G., Ode, P. J., Rendahl, A. K., Vet, L. E. M., Whitfield, J. B., Heimpel, G. E. Experimental support for Multiple-locus complementary sex determination in the parasitoid Cotesia vestalis. 遗传学. 180 (3), 1525-1535 (2008).
- Paladino, L. C., et al. Complementary sex determination in the parasitic wasp Diachasmimorpha longicaudata. PLoS ONE. 10 (3), (2015).
- Kobayashi, K., Hasegawa, E., Ohkawara, K. No gene flow between wing forms and clonal reproduction by males in the long-winged form of the ant Vollenhovia emeryi. Insectes Sociaux. 58 (2), 163-168 (2011).
- Cowan, D. P., Stahlhut, J. K. Functionally reproductive diploid and haploid males in an inbreeding hymenopteran with complementary sex determination. Proceedings of the National Academy of Sciences. 101 (28), 10374-10379 (2004).
- Armitage, S., Boomsma, J., Baer, B. Diploid male production in a leaf-cutting ant. Ecological Entomology. 35 (2), 175-182 (2010).
- Krieger, M. J. B., Ross, K. G., Chang, C. W. Y., Keller, L. Frequency and origin of triploidy in the fire ant Solenopsis invicta. Heredity. 82, 142-150 (1999).
- Seeley, T. D., Mikheyev, A. S. Reproductive decisions by honey bee colonies: Tuning investment in male production in relation to success in energy acquisition. Insectes Sociaux. 50 (2), 134-138 (2003).
- Hölldobler, B., Wilson, E. O. . The Ants. , (1990).
- da Souza, D. J., Marques Ramos Ribeiro, M., Mello, A., Lino-Neto, J., Cotta Dângelo, R. A., Della Lucia, T. M. C. A laboratory observation of nuptial flight and mating behaviour of the parasite ant Acromyrmex ameliae (Hymenoptera: Formicidae). Italian Journal of Zoology. 78 (3), 405-408 (2011).
- Woyke, J. What happens to diploid drone larvae in a honeybee colony. Journal of Apicultural Research. 2 (2), 73-75 (1963).
- Schmidt, A. M., Linksvayer, T. A., Boomsma, J. J., Pedersen, J. S. No benefit in diversity? The effect of genetic variation on survival and disease resistance in a polygynous social insect. Ecological Entomology. 36 (6), 751-759 (2011).
- Schrempf, a., Aron, S., Heinze, J. Sex determination and inbreeding depression in an ant with regular sib-mating. Heredity. 97 (1), 75-80 (2006).

