Preparation of Biomass-based Mesoporous Carbon with Higher Nitrogen-/Oxygen-chelating Adsorption for Cu(II) Through Microwave Pre-Pyrolysis
Summary
Here, we present a protocol to synthesize nitrogen/oxygen dual-doped mesoporous carbon from biomass by chemical activation in different pyrolysis modes followed by modification. We demonstrate that the microwave pyrolysis benefits the subsequent modification process to simultaneously introduce more nitrogen and oxygen functional groups on the carbon.
Abstract
An environment-friendly technique for synthesizing biomass-based mesoporous activated carbon with high nitrogen-/oxygen-chelating adsorption for Cu(II) is proposed. Bagasse impregnated with phosphoric acid is utilized as the precursor. To pyrolyze the precursor, two separate heating modes are used: microwave pyrolysis and conventional electric-heating pyrolysis. The resulting bagasse-derived carbon samples are modified with nitrification and reduction modification. Nitrogen (N)/oxygen (O) functional groups are simultaneously introduced to the surface of activated carbon, enhancing its adsorption of Cu(II) by complexing and ion-exchange. Characterization and copper adsorption experiments are performed to investigate the physicochemical properties of four prepared carbon samples and determine which heating method favors the subsequent modification for doping of N/O functional groups. In this technique, based on analyzing data of nitrogen adsorption, Fourier transform infrared spectroscopy, and batch adsorption experiments, it is proven that microwave-pyrolyzed carbon has more defect sites and, therefore, time-saving effective microwave pyrolysis contributes more N/O species to the carbon, although it leads to a lower specific surface area. This technique offers a promising route to synthesis adsorbents with higher nitrogen and oxygen content and a higher adsorption capacity of heavy-metal ions in wastewater remediation applications.
Introduction
Activated carbon has unique adsorption properties, such as a developed porous structure, a high specific surface area, and various surface functional groups; therefore, it is employed as an adsorbent in water treatment or purification1,2,3,4. Besides its physical advantages, activated carbon is cost-effective and harmless to the environment, and its raw material (e.g., biomass) is abundant and easily obtained5,6. The physicochemical properties of activated carbon depend on the precursors that are used in its preparation and on the experimental conditions of the activation process7.
Two methods are typically used to prepare activated carbon: a one-step and a two-step approach8. The term one-step approach refers to precursors being carbonized and activated simultaneously while the two-step approach refers to that sequentially. In view of energy conservation and environmental protection, the one-step approach is more preferred for its lower temperature and pressure demanding.
Besides, chemical and physical activation are utilized to improve the textural properties of activated carbon. Chemical activation possesses apparent advantages over physical activation because of its lower activation temperature, shorter activation time, higher carbon yield, and more developed and controllable pore structure in a certain degree9. It has been tested that chemical activation can be performed by impregnating biomass used as feedstock with H3PO4, ZnCl2, or other specific chemicals, followed by pyrolysis to increase the porosity of the activated carbon, because lignocellulosic components of biomass can be easily removed by a subsequent heating treatment, owing to the dehydrogenation capability of these chemicals10,11. Hence, chemical activation greatly enhances the formation of activated carbon's pores or improves the adsorptive performance to contaminants12. An acidic activator is preferred to H3PO4, due to its relatively lower energy demand, higher yield, and less impact on the environment13.
Microwave pyrolysis has the superiority in time savings, uniform interior heating, energy-efficiency, and selective heating, making it an alternative heating method to synthesis-activated carbon14,15. Compared with conventional electric heating, microwave pyrolysis can enhance thermo-chemical processes and promote certain chemical reactions16. Recently, extensive studies have focused on preparing activated carbon by chemical activation from biomass using one-step microwave pyrolysis9,17,18,19. So, it is considerably informative and environment-friendly to synthesis biomass-based activated carbon by microwave-assisted H3PO4 activation.
In addition, to improve the adsorption affinities of activated carbon toward specific heavy-metal ions, modification by heteroatom [N, O, sulfur (S), etc.] doping into carbon structures has been proposed, and this has proven to be a desirable method20,21,22,23,24,25,26. Defective sites in or at the edges of a graphite layer can be replaced by heteroatoms to generate functional groups27. Hence, nitrification and reduction modification are used to modify resultant carbon samples to dope N/O functional groups which play a crucial role in efficiently coordinating with heavy metal to form complexing and ion-exchange28.
Based on the findings above, we present a protocol to synthesize N/O dual-doped mesoporous carbon from biomass by chemical activation and two different pyrolysis methods followed up by modification. This protocol also determines which heating method favors the ensuing modification for doping of the N/O functional groups and, thus, enhancing the adsorption performance.
Protocol
1. Preparation of Bagasse-based Activated Carbon
- Preparation of the precursor for bagasse-based activated carbon
- Rinse the bagasse (obtained from a farm in Jiangsu, China) with deionized water and put the samples in a drying oven at 100 °C for 10 h.
- Crush the dried bagasse with a grinder and sieve the powder through a 50-mesh sieve.
- Place 30 g of fine bagasse powder into a 15 wt% phosphoric acid (H3PO4) solution in a 1:1 weight ratio for 24 h. Dry the mixture in an oven at 105 °C for 6 h. Collect the resulting product as the precursor for bagasse-based activated carbon (BAC).
- Conventional electric-heating pyrolysis of the precursor
- Put 15 g of the precursor into a quartz boat and then insert the quartz boat into a quartz glass tube of an electric furnace.
- Set the heating rate of the furnace at 5 °C min-1 to carbonize the sample. When the temperature reaches 500 °C, keep the temperature for 90 min and then allow the resulting activated-carbon sample to cool to room temperature in nitrogen. Ensure a nitrogen flow of 80 mL min-1 with a rotor flowmeter during the overall process.
- Triturate and collect the electrical-furnace-pyrolyzed bagasse-based activated carbon (EBAC) in a beaker and then heat it in a vacuum drying oven at 105 °C for 24 h.
- Microwave pyrolysis of the precursor
- Put 15 g of the precursor in a microwave oven (with a 2.45 GHz frequency).
- Set the power of the microwave oven at 900 W to pyrolyze the sample for 22 min, and ensure the nitrogen flow rate at 20 mL min-1 with a rotor flowmeter. The air inlet of the rotor flowmeter is connected to a nitrogen cylinder using a hose, while the outlet is connected to the air inlet of the microwave oven.
- Allow the resultant carbon to cool to room temperature in nitrogen. Triturate and collect the carbon sample in a beaker and then add 300 mL of hydrochloric acid (0.1 M). Stir the mixture using a magnetic stirrer (at 200 rpm) for more than 12 h at room temperature.
- Filter the carbon by filter paper with vacuum filtration and rinse the sample with deionized water until the pH value of the wash water is > 6. Dry the microwave-pyrolyzed bagasse-based activated carbon (MBAC) in a vacuum drying oven at 105 °C for 24 h.
2. Modification of Electrical-furnace-pyrolyzed Bagasse-based Activated Carbon and Microwave-pyrolyzed Bagasse-based Activated Carbon
Note: The modification of the two samples was conducted according to the literature29.
- Nitrification
- Mix 50 mL of concentrated sulfuric and 50 mL of concentrated nitric acids in a beaker at 0 °C (in an ice bath).
CAUTION: When the mixture of concentrated sulfuric acid and concentrated nitric acid is mixed, the concentrated sulfuric acid should be slowly added to the concentrated nitric acid and stirred with a glass rod and cooled in time. - Add 10 g of EBAC/MBAC to the mixed solution. Use a magnetic stirrer to stir the mixture for 120 min (at 200 rpm).
- Filter the nitrified EBAC/MBAC by filter paper with vacuum filtration. Wash the carbon with deionized water until the wash water reaches pH 6, and then dry it in a drying oven at 90 °C for 24 h.
- Mix 50 mL of concentrated sulfuric and 50 mL of concentrated nitric acids in a beaker at 0 °C (in an ice bath).
- Reductive modification
- In a three-necked flask, add the 5.05 g of resulting product, 50 mL of deionized water, and 20 mL of ammonia solution (15 M). Stir this mixture for 15 min with a magnetic stirrer (at 200 rpm), then add 28 g of Na2S2O4, and leave the mixture stirring at room temperature for 20 h.
- Fit a reflux condenser to the flask and warm the mixture up to 100 °C using an oil bath. Add 120 mL of CH3COOH (2.9 M) to the flask and allow the mixture to stir for 5 h with a magnetic stirrer (at 200 rpm) under reflux.
- Remove the oil bath to allow the solution to cool down to room temperature. Filter the carbon sample and wash it with deionized water until the solution pH > 6. Dry the modified EBAC/MBAC at 90 °C and denote it as “EBAC-N/MBAC-N”.
3. Adsorbent Characterization
- Structural characterization—Nitrogen adsorption/desorption isotherms
- Weigh an empty sample tube. Add a carbon sample (~0.15 g) to the sample tube.
- Degas the sample at 110 °C for 5 h in a vacuum. Weigh the sample tube containing carbon. Calculate the weight of the carbon sample.
- Install the sample tube into the test area of the surface-area and porosimetry analyzer using liquid nitrogen to measure it at -196 °C30.
- Chemical characterization—Fourier transform infrared spectroscopy
- Check the temperature and hygrometer and observe whether the environment meets the requirements: the temperature should be 16 – 25 °C and the relative humidity 20% – 50%.
- Remove the desiccant and dust cover in the sample storehouse.
- Dry the carbon sample and potassium bromide at 110 °C for 4 h to avoid the effect of water on the spectrum. Mix the carbon sample with potassium bromide and then use a press mechanism to prepare the test sample.
- Put the sample in the test area and set the parameters of the software.
- Save the spectra and take out the sample. Perform a required data processing for the spectra31.
4. Cu(II)-adsorption Experiments
- Adsorption isotherm
- Place 0.05 g of adsorbent in each of the conical flasks, which contain 25 mL of a CuSO4 solution (pH 5) with a selected initial concentration (10, 20, 30, 40, 50, 60, 80, and 100 mg L-1). Use a 0.1 M HNO3 and 0.1 M NaOH solution to adjust the pH of each copper solution.
Note: A solution with the selected initial concentration is diluted by a 1 g L-1 CuSO4 solution, which is made up of a dissolved 3.90625 g of blue vitriol solid using the vase with a 1,000 mL volume. - Fit lids on the conical flasks and put them in a thermostatic orbital shaker (with a stirring rate of 150 rpm) at 5 °C/25 °C/45 °C for 240 min.
- Use 0.22 μm membrane filters to separate the adsorbents from the solution.
- Use a flame atomic absorption spectrophotometry to determine the copper concentration of the filtrate.
Note: All experiments were carried out in triplicate and the data were averaged. The adsorption capacity for Cu(II), qe, was calculated as follows:
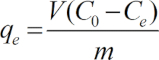 (1)
(1)
Here,
C0 = the initial copper concentration (mg L-1),
Ce = the final concentration (mg L-1),
V = the solution volume, and
m = the weight of each adsorbent (g).
- Place 0.05 g of adsorbent in each of the conical flasks, which contain 25 mL of a CuSO4 solution (pH 5) with a selected initial concentration (10, 20, 30, 40, 50, 60, 80, and 100 mg L-1). Use a 0.1 M HNO3 and 0.1 M NaOH solution to adjust the pH of each copper solution.
- Influence of pH
- Place 0.05 g of adsorbent in each of conical flasks, which contain 25 mL of a CuSO4 solution (40 mg L-1) with a selected initial pH (2, 3, 4, 5, 6, and 7).
- Fit lids on the conical flasks and put them in a thermostatic orbital shaker (with a stirring rate of 150 rpm) at 25 °C for 24 h to reach adsorption equilibrium conditions.
- Repeat step 4.1.3-4.1.4.
- Adsorption kinetics
- Place 0.25 g of adsorbent in a beaker which contains 125 mL of a CuSO4 solution (30 mg L-1 or 100 mg L-1, pH 5) in a 25 °C water bath with magnetic stirring (at 200 rpm).
- Use pipettes to draw 5 mL of the solution when the contact time reaches 0.5, 1, 2.5, 5, 10, 30, 60, 120, and 180 min.
- Repeat step 4.1.3-4.1.4.
Representative Results
Nitrogen adsorption/desorption isotherms of four samples are presented in Figure 1. All adsorption isotherms show a rapid increase in low P/P0 region and these isotherms belong to type IV (IUPAC classification) demonstrating their pore structure that consists of micropores and dominant mesopores32.
The surface physical parameters for all samples obtained from the nitrogen adsorption isotherms are shown in Table 1. Microwave pyrolysis and modification both contribute to a smaller Brunauer-Emmett-Teller (BET) surface area and total pore volume, changing the physical morphology of the samples.
Fourier transform infrared (FTIR) spectra of the four samples are given in Figure 2. Bands of MBAC at 1167 cm-1 [carbon (C) – O stretching vibration], 1620 cm-1 (C = O stretching vibration), 2852 cm-1 [N – hydrogen (H) stretching vibration], 2922 cm-1 (C – H stretching vibration), and 3442 cm-1 (O – H stretching vibration) are more intense than EBAC. These may be attributed to the microwave pyrolysis contributing more oxygen functional groups to the BAC surface. For EBAC-N and MBAC-N, bands around 1573 cm-1 and 1400 cm-1 likely represent C = N and N – H groups, respectively. It can be found that the modified carbon materials have obtained distinct nitrogen/oxygen functional groups, and the microwave-pyrolyzed carbon gets more, which is in accordance with the elemental analysis as shown in Table 1. It can be speculated that microwave pyrolysis is more adequate to activate the precursor and lay root for further modifications than conventional electric-heating pyrolysis. MBAC-N possesses mainly hydroxyl, carboxyl, amino, and imine functional groups.
Figure 3 shows the adsorption capacity of the four samples under different pH conditions. The four adsorbents reach the optimal adsorption capacity at pH 5, so the following adsorption experiments are all carried out at pH 5. The samples prepared by microwave pyrolysis exhibited better Cu(II) adsorption capacity before and after the modification, although they had a lower specific surface area and pore volume. In general, the adsorbability of adsorbents depends on the pore structure and surface functional groups. Therefore, the high adsorption capacity of MBAC-N is attributed to more abundant N/O surface groups. The results confirm that the microwave pyrolysis benefits the follow-up introduction of surface functional groups to improve the adsorption capacity more than electric-heating pyrolysis.
The adsorption isotherms of MBAC-N on Cu(II) at 5 °C, 25 °C, and 45 °C are shown in Figure 4a. The adsorption properties of samples for Cu(II) become better when the temperature increases. By comparing the isotherm parameters in Table 2, it is clear that the Langmuir isotherm model indicates a higher linear correlation coefficient (R2) which is over 0.99 (the fitting line in Figure 4b), and the measured adsorption capacity (q0mea) is identical with the calculated one (q0cal). Therefore, the model is more suitable than the Freundlich and Temkin isotherm models, which indicates that the absorption of Cu(II) is a chemical adsorption process33.
As shown in Figure 4c, MBAC-N can reach about 75% of Cu(II) equilibrium adsorption capacity within 15 min, and it can almost reach the adsorption equilibrium of Cu(II) in about 50 min at different initial concentrations. These prove that MBAC-N has excellent adsorption properties. As can be seen from Table 3, the pseudo-second-order model is better than the Lagergren and Elovich models with R2 = 0.999 (the fitting line in Figure 4d). The above results confirm that the adsorption of Cu(II) on MBAC-N is chemisorption. Hence, the chemical interaction mechanism of Cu(II) by the modified carbon is proposed in Figure 5. Table 4 compares the adsorption capacity of Cu(II) by biomass-based activated carbon reported in recent references34,35,36,37,38. It is found that MBAC-N has a higher adsorption capacity than other adsorbents reported in the literature, demonstrating it as a promising adsorbent for removing Cu(II).
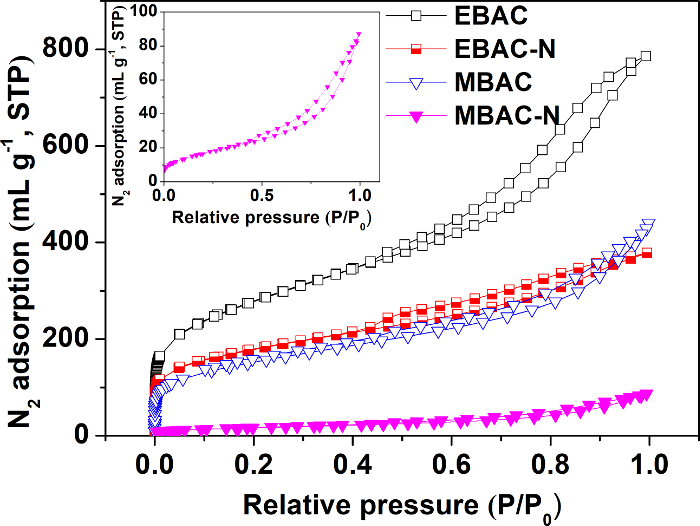
Figure 1: Nitrogen adsorption/desorption isotherms of carbons. The inset graph in Figure 1 shows the nitrogen adsorption/desorption isotherm of MBAC-N in a smaller ordinate range. The data were obtained from the supporting software of the surface-area and porosimetry analyzer. This figure has been modified from Wan and Li27. Please click here to view a larger version of this figure.
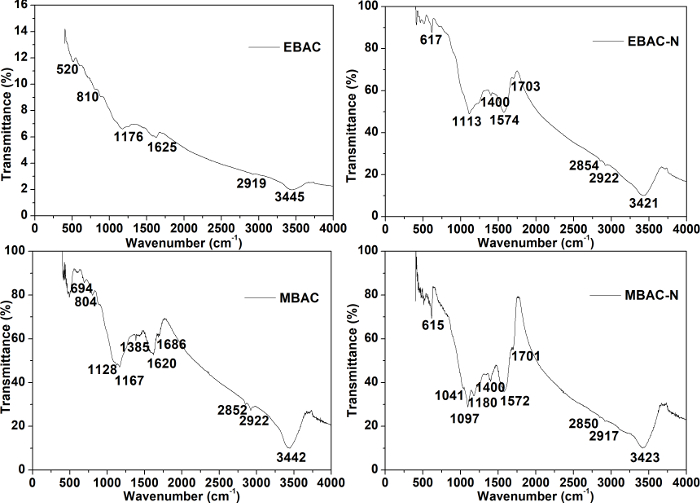
Figure 2: FTIR spectra of EBAC, EBAC-N, MBAC, and MBAC-N. The spectra can confirm the chemical compositions and surface functional groups of the samples. This figure has been modified from Wan and Li27. Please click here to view a larger version of this figure.
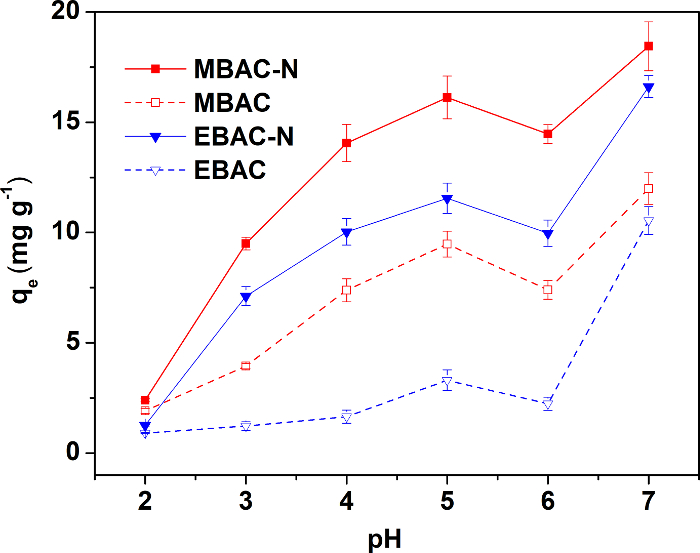
Figure 3: Effect of solution pH on Cu(II) adsorption. The concentration of copper in the solutions is 40 mg L-1. The test is conducted at 25 °C and at 150 rpm for 24 h, to reach adsorption equilibrium. This figure has been modified from Wan and Li27. Please click here to view a larger version of this figure.
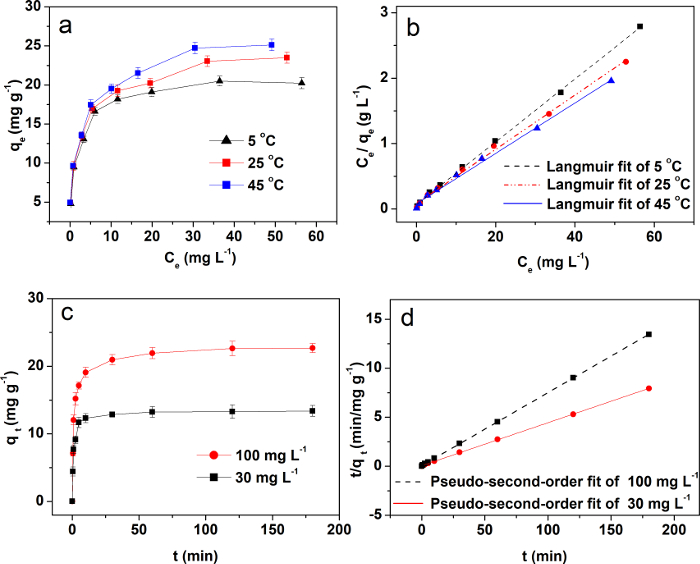
Figure 4: Representative adsorption property analysis of MBAC-N. (a) This panel shows the adsorption isotherms of Cu(II) on MBAC-N at 5 °C, 25 °C, and 45 °C. (b) This panel shows the fitting result for copper adsorption by using the Langmuir isotherm. (c) This panel shows the kinetics of Cu(II) on MBAC-N at the initial concentrations of 30 mg L-1 and 100 mg L-1. (d) This panel shows the fitting result for copper adsorption at 25 °C by using the Pseudo-second-order model. This figure has been modified from Wan and Li27. Please click here to view a larger version of this figure.
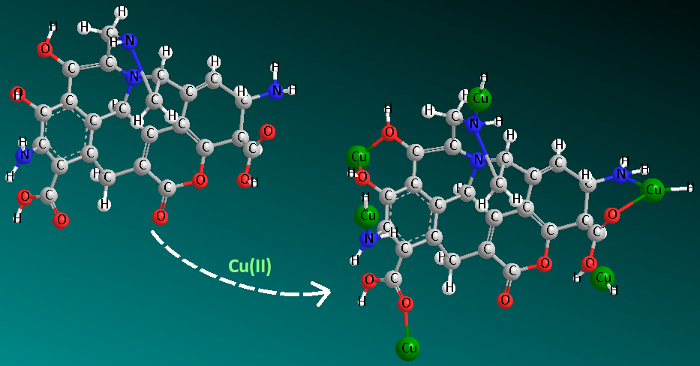
Figure 5: Proposed mechanism for Cu(II) adsorption by modified carbon. In this reaction process, the chemical adsorption mainly involves ion exchange and complexing. Please click here to view a larger version of this figure.
| Adsorbents | EBAC | EBAC-N | MBAC | MBAC-N |
| Pore structure parameters | ||||
| BET surface area (m2 g−1) | 978 | 609 | 543 | 61 |
| Total pore volume (cm3 g−1) | 1.22 | 0.59 | 0.68 | 0.13 |
| Mesoporous volume (cm3 g−1) | 1.09 | 0.47 | 0.58 | 0.11 |
| Mean pore size DP (nm) | 4.97 | 3.84 | 5.01 | 8.89 |
| Mesoporous rate (%) | 89.52 | 80.24 | 85.32 | 84.61 |
| Elemental content (wt%) | ||||
| C | 92.23 | 79.31 | 87.28 | 72.44 |
| H | 1.76 | 1.26 | 1.65 | 1.12 |
| N | 0.08 | 4.01 | 0.58 | 5.52 |
| O | 5.82 | 15.15 | 10.33 | 20.54 |
| S | 0.11 | 0.27 | 0.16 | 0.38 |
| Yield (%) | 53.35 | / | 57.23 | / |
Table 1: Structural characteristics and elemental compositions of EBAC, EBAC-N, MBAC, and MBAC-N. The textural data are analyzed using the BET method. The relative weight percentage of the elements is calculated based on the dry ash-free basis. This table has been modified from Wan and Li27.
| MBAC-N | ||||
| Isotherm models | Parameters | 5 °C | 25 °C | 45 °C |
| Langmuir | q0cal (mg g−1) | 20.82 | 24.09 | 25.97 |
| q0mea (mg g−1) | 20.23 | 23.47 | 25.12 | |
| b (L mg−1) | 0.73 | 0.51 | 0.49 | |
| R2 | 0.999 | 0.996 | 0.995 | |
| Freundlich | KF (L mg−1) | 8.802 | 9.65 | 10.56 |
| n | 3.937 | 3.902 | 4.032 | |
| R2 | 0.907 | 0.967 | 0.987 | |
| Temkin | AT (L mg−1) | 29.57 | 32.3 | 49.8 |
| B (L mg−1) | 2.94 | 3.19 | 3.16 | |
| R2 | 0.969 | 0.985 | 0.955 | |
Table 2: Isotherm parameters of Cu(II) on MBAC-N at different temperatures. The fitted parameters are from linearized Langmuir, Freundlich, and Temkin adsorption models. This table has been modified from Wan and Li27.
| MBAC-N | |||
| Kinetic models | Parameters | 30 mg L−1 | 100 mg L−1 |
| Lagergren | k1 (min−1) | 0.037 | 0.045 |
| R2 | 0.714 | 0.934 | |
| qe,mea (mg g−1) | 13.39 | 22.69 | |
| Pseudo-second-order | qe,cal (mg g−1) | 13.44 | 23.25 |
| k2 (g (mg min)−1) | 0.08676 | 0.03031 | |
| R2 | 0.999 | 0.999 | |
| qe,mea (mg g−1) | 13.39 | 22.69 | |
| Elovich | αE (g (mg min)−1) | 379.73 | 312.25 |
| βE (mg g−1) | 0.738 | 0.411 | |
| R2 | 0.799 | 0.901 | |
Table 3: Kinetic parameters of Cu(II) on MBAC-N at different initial concentrations. The fitted parameters are from linearized Lagergren, Pseudo-second-order, and Elovich models. This table has been modified from Wan and Li27.
| Adsorbents | pH | qe (mg g−1) | References |
| Wood-based granular activated carbon | 5.5 | 6.016 | 34 |
| Baobab fruit shell-derived activated carbon | 6 | 3.0833 | 35 |
| Olive stone AC (COSAC) | 5 | 17.08 | 36 |
| Activated carbonfrom date stones | 5.5 | 18.68 | 37 |
| Walnut shell based activated carbon | 5 | 9.3 | 38 |
| Plasma modified activated carbon | 21.4 | ||
| MBAC-N | 5 | 25.12 | This study |
Table 4: Comparison of the adsorption capacity of Cu(II) on different adsorbents. The ability of activated carbon to remove Cu(II) is significantly affected by the pH of the solution, so the adsorption capacity of the contrast biomass-based carbon materials should be obtained close to pH 5.
Discussion
In this protocol, one of the critical steps is the successful preparation of mesoporous carbon with better physicochemical properties by the one-step approach, where optimal experimental conditions need to be determined. So, in a previous study28, we have carried out orthogonal array microwave pyrolysis experiments, considering the effect of the impregnation ratio of bagasse and phosphoric acid, pyrolysis time, microwave oven power, and drying time. Besides, great care must be taken in tedious Cu(II)-adsorption experiments, especially when the pH value of the solution is adjusted, because the pH value has a great influence on Cu(II) removal by activated carbon (Figure 3). It is imperative to test the actual copper concentration of the CuSO4 solution with a defined initial concentration and use this value as C0 in Equation (1).
A larger specific surface area and higher pore volume of biomass-based activated carbon can be obtained by chemical activation. However, the specific surface area and total pore volume both decrease during the subsequent pyrolysis and modification process, which is likely due to the collapse and blockage of the pores27, resulting in a reduction of the adsorption capacity. Therefore, further work is required to prepare biomass-based mesoporous carbon with both a high surface area and abundant functional groups.
Microwave pyrolysis is verified to more adequately synthesize biomass-based mesoporous carbon with a higher nitrogen/oxygen-chelating adsorption for Cu(II), which has many advantages over widely used conventional heating methods. However, it is not possible to control the instantaneous temperature accurately during the microwave pyrolysis process. Biomass is a good microwave absorption material, whose temperature can rapidly increase under the effect of a microwave. Clearly, future work needs to examine how the pyrolysis temperature affects the physicochemical properties of the biomass-based carbon.
A detailed description of the modification mechanism is beyond the scope of this article, but it can be found in earlier published literature27. The potential importance of nitrification and reduction modification which can effectively introduce more N/O functional groups concurrently on the surface of carbon samples is worth appreciating. However, the modification process contains numerous experimental steps and the utilization of dangerous concentrated strong acid. A simpler and more effective nitrogen/oxygen modification method may be tested and adopted in further studies.
We have demonstrated an environment-friendly energy-efficient method for preparing biomass-based mesoporous carbon by microwave pyrolysis and dope N/O groups simultaneously on the carbon using a nitrification and reduction route. Such N/O dual-doped activated carbon owns a higher adsorption capacity of heavy-metal ions in an aqueous solution, which is applicable for wastewater remediation. We expect that this protocol will provide ideas for the rapid preparation of high-adsorptive carbon from biomass by time-saving, effective microwave pyrolysis and will be optimized in the future.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge the Fundamental Research Funds for the Central Universities of China (No.KYZ201562), China Postdoctoral Science Fund (No. 2014M560429) and the Key research and development plan of Jiangsu Province (No. BE2018708).
Materials
| All chemicals and reagents (phosphoric acid, etc.) | Nanjing Chemical Reagent Co., Ltd | Analytical grade | |
| Electric furnace | Luoyang Bolaimaite Experiment Electric Furnace Co., Ltd | ||
| Microwave oven | Nanjing Yudian Automation Technology Co., Ltd | 2.45 GHz frequency | |
| Surface-area and porosimetry analyzer | Beijing Gold APP Instrument Co., Ltd | Vc-Sorb 2800TP | |
| Fourier transform infrared (FTIR) spectrometer | Nicolet | 6700 | |
| Flame atomic absorption spectrophotometry | Beijing Purkinje General Instrument Corporation | A3 | |
| Element Analyzer | Germany Heraeus Co. | CHN-O-RAPID |
References
- Saleh, T. A., Gupta, V. K. Processing methods, characteristics and adsorption behavior of tire derived carbons: a review. Advances in Colloid & Interface Science. 211, 93 (2014).
- Mohammadi, N., Khani, H., Gupta, V. K., Amereh, E., Agarwal, S. Adsorption process of methyl orange dye onto mesoporous carbon material-kinetic and thermodynamic studies. Journal of Colloid & Interface Science. 362 (2), 457 (2011).
- Saleh, T. A., Gupta, V. K. Column with CNT/magnesium oxide composite for lead(II) removal from water. Environmental Science & Pollution Research. 19 (4), 1224-1228 (2012).
- Asfaram, A., Ghaedi, M., Agarwal, S., Tyagi, I., Kumargupta, V. Removal of basic dye Auramine-O by ZnS:Cu nanoparticles loaded on activated carbon: optimization of parameters using response surface methodology with central composite design. RSC Advances. 5 (24), 18438-18450 (2015).
- Gupta, V. K., Saleh, T. A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene- an overview. Environmental Science and Pollution Research. 20 (5), 2828-2843 (2013).
- Ahmaruzzaman, M., Gupta, V. K. Rice Husk and Its Ash as Low-Cost Adsorbents in Water and Wastewater Treatment. Industrial & Engineering Chemistry Research. 50 (24), 13589-13613 (2011).
- Ahmed, M. J., Theydan, S. K. Adsorption of cephalexin onto activated carbons from Albizia lebbeck seed pods by microwave-induced KOH and K2CO3 activations. Chemical Engineering Journal. 211 (22), 200-207 (2012).
- Liew, R. K., et al. Production of activated carbon as catalyst support by microwave pyrolysis of palm kernel shell: a comparative study of chemical versus physical activation. Research on Chemical Intermediates. , 1-17 (2018).
- Lam, S. S., et al. Microwave-assisted pyrolysis with chemical activation, an innovative method to convert orange peel into activated carbon with improved properties as dye adsorbent. Journal of Cleaner Production. 162, 1376-1387 (2017).
- Jin, H., Wang, X., Gu, Z., Polin, J. Carbon materials from high ash biochar for supercapacitor and improvement of capacitance with HNO3 surface oxidation. Journal of Power Sources. 236, 285-292 (2013).
- Chen, H. Research Methods for the Biotechnology of Lignocellulose. Biotechnology of Lignocellulose: Theory and Practice. , 403-510 (2014).
- Sayğılı, H., Güzel, F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: process optimization, characterization and dyes adsorption. Journal of Cleaner Production. 113, 995-1004 (2016).
- Cao, Q., Xie, K. C., Lv, Y. K., Bao, W. R. Process effects on activated carbon with large specific surface area from corn cob. Bioresource Technology. 97 (1), 110-115 (2006).
- Xiao, X., et al. Adsorption behavior of phenanthrene onto coal-based activated carbon prepared by microwave activation. Korean Journal of Chemical Engineering. 32 (6), 1129-1136 (2015).
- Ge, X., et al. Adsorption of naphthalene from aqueous solution on coal-based activated carbon modified by microwave induction: Microwave power effects. Chemical Engineering & Processing Process Intensification. 91, 67-77 (2015).
- Yao, S., et al. Removal of Pb(II) from water by the activated carbon modified by nitric acid under microwave heating. Journal of Colloid and Interface Science. 463, 118-127 (2016).
- Ali, A., Idris, R. Utilization Of Low-cost Activated Carbon From Rapid Synthesis Of Microwave Pyrolysis For WC Nanoparticles Preparation. Advanced Materials Letters. 08 (1), 70-76 (2016).
- Puchana-Rosero, M. J., et al. Microwave-assisted activated carbon obtained from the sludge of tannery-treatment effluent plant for removal of leather dyes. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 504, 105-115 (2016).
- Du, Z. L., Zheng, T., Wang, P., Hao, L. L., Wang, Y. X. Fast microwave-assisted preparation of a low-cost and recyclable carboxyl modified lignocellulose-biomass jute fiber for enhanced heavy metal removal from water. Bioresource Technology. 201, 41-49 (2016).
- Ge, X., et al. Microwave-assisted modification of activated carbon with ammonia for efficient pyrene adsorption. Journal of Industrial & Engineering Chemistry. 39, 27-36 (2016).
- Ghaedi, M., et al. Modeling of competitive ultrasonic assisted removal of the dyes – Methylene blue and Safranin-O using Fe3O4 nanoparticles. Chemical Engineering Journal. 268, 28-37 (2015).
- Gupta, V. K., Nayak, A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chemical Engineering Journal. 180 (3), 81-90 (2012).
- Robati, D., et al. Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chemical Engineering Journal. 284 (7), 687-697 (2016).
- Ali, I., Alothman, Z. A., Sanagi, M. M. Green Synthesis of Iron Nano-Impregnated Adsorbent for Fast Removal of Fluoride from Water. Journal of Molecular Liquids. 211, 457-465 (2015).
- Gupta, V. K., Kumar, R., Nayak, A., Saleh, T. A., Barakat, M. A. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review. Advances in Colloid & Interface Science. 193 (6), 24 (2013).
- Mittal, A., Mittal, J., Malviya, A., Gupta, V. K. Adsorptive removal of hazardous anionic dye "Congo red" from wastewater using waste materials and recovery by desorption. Journal of Colloid and Interface Science. 340 (1), 16-26 (2009).
- Wan, Z., Li, K. Effect of pre-pyrolysis mode on simultaneous introduction of nitrogen/oxygen-containing functional groups into the structure of bagasse-based mesoporous carbon and its influence on Cu(II) adsorption. Chemosphere. 194, 370-380 (2018).
- Li, K., Li, J., Lu, M., Li, H., Wang, X. Preparation and amino modification of mesoporous carbon from bagasse via microwave activation and ethylenediamine polymerization for Pb(II) adsorption. Desalination and Water Treatment. 57 (50), 24004-24018 (2016).
- Yantasee, W., et al. Electrophilic Aromatic Substitutions of Amine and Sulfonate onto Fine-Grained Activated Carbon for Aqueous-Phase Metal Ion Removal. Separation Science and Technology. 39 (14), 3263-3279 (2004).
- Li, Y. B., Li, K. Q., Wang, X. H., Li, J. Ethylenediamine Modification of Hierarchical Mesoporous Carbon for the Effective Removal of Pb (II) and Related Influencing Factors. International Journal of Material Science. 6 (1), 58-65 (2016).
- Georgakopoulos, E., Santos, R. M., Chiang, Y. W., Manovic, V. Two-way Valorization of Blast Furnace Slag: Synthesis of Precipitated Calcium Carbonate and Zeolitic Heavy Metal Adsorbent. Journal of Visualized Experiments. (120), e55062 (2017).
- Loganathan, P., et al. Modelling equilibrium adsorption of single, binary, and ternary combinations of Cu, Pb, and Zn onto granular activated carbon. Environmental Science & Pollution Research. (15), 1-12 (2018).
- Vunain, E., Kenneth, D., Biswick, T. Synthesis and characterization of low-cost activated carbon prepared from Malawian baobab fruit shells by H3PO4 activation for removal of Cu(II) ions: equilibrium and kinetics studies. Applied Water Science. 7 (8), 4301-4319 (2017).
- Bohli, T., Ouederni, A., Villaescusa, I. Simultaneous adsorption behavior of heavy metals onto microporous olive stones activated carbon: analysis of metal interactions. Euro-Mediterranean Journal for Environmental Integration. 2 (1), 19 (2017).
- Bouhamed, F., Elouear, Z., Bouzid, J., Ouddane, B. Multi-component adsorption of copper, nickel and zinc from aqueous solutions onto activated carbon prepared from date stones. Environmental Science & Pollution Research. 23 (16), 1-6 (2016).
- Wu, L., et al. Surface modification of phosphoric acid activated carbon by using non-thermal plasma for enhancement of Cu(II) adsorption from aqueous solutions. Separation & Purification Technology. 197, (2018).

