NMR Spectroscopy as a Robust Tool for the Rapid Evaluation of the Lipid Profile of Fish Oil Supplements
Summary
Here, high-resolution 1H and 13C Nuclear Magnetic Resonance (NMR) spectroscopy was used as a rapid and reliable tool for quantitative and qualitative analysis of encapsulated fish oil supplements.
Abstract
The western diet is poor in n-3 fatty acids, therefore the consumption of fish oil supplements is recommended to increase the intake of these essential nutrients. The objective of this work is to demonstrate the qualitative and quantitative analysis of encapsulated fish oil supplements using high-resolution 1H and 13C NMR spectroscopy utilizing two different NMR instruments; a 500 MHz and an 850 MHz instrument. Both proton (1H) and carbon (13C) NMR spectra can be used for the quantitative determination of the major constituents of fish oil supplements. Quantification of the lipids in fish oil supplements is achieved through integration of the appropriate NMR signals in the relevant 1D spectra. Results obtained by 1H and 13C NMR are in good agreement with each other, despite the difference in resolution and sensitivity between the two nuclei and the two instruments. 1H NMR offers a more rapid analysis compared to 13C NMR, as the spectrum can be recorded in less than 1 min, in contrast to 13C NMR analysis, which lasts from 10 min to one hour. The 13C NMR spectrum, however, is much more informative. It can provide quantitative data for a greater number of individual fatty acids and can be used for determining the positional distribution of fatty acids on the glycerol backbone. Both nuclei can provide quantitative information in just one experiment without the need of purification or separation steps. The strength of the magnetic field mostly affects the 1H NMR spectra due to its lower resolution with respect to 13C NMR, however, even lower cost NMR instruments can be efficiently applied as a standard method by the food industry and quality control laboratories.
Introduction
The consumption of n-3 fatty acids in the diet has proven to be beneficial against several conditions such as heart disorders1,2,3, inflammatory diseases4 and diabetes5. The Western diet is considered poor in n-3 fatty acids and thus the consumption of fish oil supplements is recommended to improve the n-6/n-3 balance in consumer's nutrition1. Despite the recent increase in fish oil supplement consumption, questions remain about the safety, authenticity, and quality of some of these products. The rapid and accurate compositional analysis of fish oil supplements is essential to properly evaluate the quality of these commercial products and ensure consumer safety.
The most common methodologies for the assessment of fish oil supplements are gas chromatography (GC) and Infrared Spectroscopy (IR). While these are highly sensitive methods, they suffer from several drawbacks6. GC analysis is time consuming (4-8 h) because separation and derivatization of individual compounds is required7 and lipid oxidation may occur during the analysis8,9. While IR spectroscopy can be quantitative, a prediction model is required to be constructed using partial least squares regression (PLSR), although there are exceptions in which IR bands can be attributed to a single compound10. PLSR requires the analysis of a large number of samples, which increases the time of the analysis11. For this reason, there is an increasing interest in the development of new analytical methodologies that allow accurate and fast analysis of a large number of fish oil samples. Organizations such as the Office of Dietary Supplements (ODS) at the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) have collaborated with the Association of Official Analytical Chemists (AOAC) to develop these new methods12,13.
One of the most promising analytical methods for the screening and the evaluation of multi-component matrices, such as dietary supplements, is Nuclear Magnetic Resonance (NMR) spectroscopy14,15. NMR spectroscopy has several advantages: it is a non-destructive and quantitative technique, it requires minimal to no sample preparation, and it is characterized by excellent accuracy and reproducibility. In addition, NMR spectroscopy is an environmentally friendly methodology because it utilizes only small amounts of solvents. The main drawback of NMR spectroscopy is its relatively low sensitivity compared to other analytical methods, however, recent technological advances in instrumentation such as stronger magnetic fields, cryogenic probes of various diameters, advanced data processing, and versatile pulse sequences and techniques have increased the sensitivity up to the nM range. While NMR instrumentation is high cost, the long-life of NMR spectrometers and the many applications of NMR lower the cost of the analysis in the long run. This detailed video protocol is intended to help new practitioners in the field avoid pitfalls associated with 1H and 13C NMR spectroscopic analysis of fish oil supplements.
Protocol
1. NMR Sample Preparation
Note: Caution, please consult all relevant material safety data sheets (MSDS) before use. Deuterated chloroform (CDCl3) used in sample preparation is toxic. Please use all the appropriate safety practices when performing sample preparation including the use of a fume hood and personal protective equipment (safety glasses, gloves, lab coat, full length pants, closed-toe shoes).
- Preparation of 1H and 13C samples
- Extract 120 µL (~ 110 mg) of fish oil from a dietary capsule using a syringe and place it in a 4 mL glass vial. Record the weight of the fish oil.
- Sample dissolution
- Dissolve approximately 120 µL of fish oil in 500 µL of CDCl3 containing 0.01% of tetramethylsilane (TMS) which is used as a reference for the 1H and 13C chemical shifts.
NOTE: TMS is used only for chemical shift calibration (see step numbers 2.2.1.2.7 and 2.2.2.2.7), not for quantification (see step numbers 2.2.1.3 and 2.2.2.3) purposes. - Prepare a 2,6-di-tert-butyl-4-methylphenol (BHT) stock solution, if quantification expressed in mg/g is desired, by dissolving approximately 220 mg of BHT and 15 mg of Chromium(III) acetylacetonate (Cr(acac)3) in 20 mL of CDCl3 containing 0.01% of TMS. Use 500 µL of the stock solution to dissolve 100 mg (± 10 mg) of fish oil.
- Dissolve approximately 120 µL of fish oil in 500 µL of CDCl3 containing 0.01% of tetramethylsilane (TMS) which is used as a reference for the 1H and 13C chemical shifts.
- After dissolving the oil (this takes a few seconds), transfer all of the solution directly into a high quality 5-mm NMR tube and attach a cap. Analyze the samples within 24 h after preparing the samples.
2. NMR Instrument preparation
Note: Caution, beware that the presence of strong magnetic fields produced by NMR instruments can affect medical devices and implants such as pacemakers and surgical prostheses, as well as electronic items such as credit cards, watches, etc. Additional caution is required when the analysis is performed using non-shielding magnets. Two NMR instruments were used for the acquisition of 1H and 13C NMR spectra; a spectrometer operating at 850.23 MHz and 213.81 MHz for 1H and 13C nuclei, respectively, equipped with a triple resonance helium-cooled inverse (TCI) 5 mm probe and a spectrometer operating at 500.20 MHz and 125.77 MHz for 1H and 13C nuclei, respectively, equipped with a broad band observed (BBO) nitrogen-cooled 5 mm probe. All experiments were performed at 25 ± 0.1 ºC and the spectra were processed by a standard NMR data analysis acquisition and processing software package (see Materials List).
- Preparation for acquiring the NMR spectra
Note: 1H and 13C NMR spectra can be acquired consequently without removing the sample from the instrument.- Insert the NMR tube into a spinner turbine (see Materials List).
- Place the spinner and the tube on the top of a graded depth gauge and gently push the top of the tube until its bottom part touches the bottom of the gauge.
- Place NMR sample in an open spot of the SampleCase. Note the slot number the sample is placed in.
- To load the sample in the NMR, return to the control computer and type 'sx #', where # is the slot in the SampleCase holding your sample.
- Wait for the deuterium signal of CDCl3 to appear on the lock window screen. If it does not automatically appear, type "lockdisp". As soon as the deuterium signal is visible, type "lock" on the command line and select "CDCl3" from the solvent's list in order to lock the sample using the CDCl3 deuterium resonance.
NOTE: Deuterium signal may not appear if previous user used a different solvent. User should wait for the indicator that the sample is down, then lock. - Type "bsmsdisp" in the command line to ensure spinning is not active. If the "SPIN" button is green, click it to deactivate spinning.
- Type the "new" command to create a new data set. Enter a name for the data set in the "NAME" tab and the experiment number in the "EXPNO" tab. Use number "1" in the "PROCNO" tab. In the "Experiment" tab, hit "Select" and choose the "PROTON" parameter file. Write the title of the experiment in the "TITLE" tab. Click "OK."
- Type "getprosol" in the command line to obtain the standard parameters for the current NMR probe and solvent.
- Repeat step 2.1.7 for 13C, selecting the "C13IG" pulse sequence in the "Experiment" tab for the 1D 13C inverse gated decoupled experiment.
- Type "getprosol" in the command line to obtain the standard parameters for the current NMR probe and solvent.
- Type the command "atma" to perform automatic tuning and matching of the probe for both carbon and proton nuclei.
- Perform one-dimensional gradient shimming to achieve a highly homogeneous magnetic field, and thus optimum line shape for the NMR signals.
- Use the standard automatic procedure for 1D shimming, simply by sequentially executing the commands "qu topshim 1dfast ss", "qu topshim tuneb ss," and "qu topshim report" on the command line.
- Parameter optimization
- 90° pulse calibration
- Create a new data set for 1H (see steps 2.1.7 and 2.1.8).
- Type the command "paropt" on the command line to start the automation program for calibrating the 90° pulse. Select pulse duration, p1, as the parameter to be modified.
- Start with "2" µs as the initial value of p1, enter "2" µs increments and perform "16" experiments.
- Create a new data set for 13C (see step 2.1.9) and repeat the process for 13C nuclei (see steps 2.2.1.2 and 2.2.1.3).
- T1 measurement measured by the null method16 for 1H
NOTE: The null method uses the inversion recovery pulse sequence, consisting of a 180° pulse follow by a delay (tau), to allow relaxation along the z axis and a final 90° pulse which creates the observable transverse magnetization.- Create a new data set for 1H (see steps 2.1.7 and 2.1.8).
- Type "pulprog t1ir1d" to change the pulse sequence to the inversion-recovery experiment.
- Type the following commands on the command line to set up the spectral width in ppm, the center of the RF transmitter, the number of scans the number of dummy scans and the number of data points "sw 8", "o1p 3.8", "ns 2", "ds 2" and "td 64K".
- Type "p1 (value)" and enter the duration values for 90° pulse as determined by the pulse calibration (see step 2.2.1) and type "p2 (value)" for the 180° pulse (the duration value for the 180° pulse is the 90° pulse duration multiplied by two).
- Set the recycle delay to a very large value, such as 10 s by typing "d1 10".
- Set tau to a short value, such as 10 ms, by typing "d7 10ms" in the command line.
- Set the receiver gain (RG) to an appropriate value using the command "rga" for automatic calculation of RG.
- Run a spectrum by typing the command "zg".
- Execute Fourier-transformation by typing "efp" in the command line.
- Perform automatic phase correction by typing the command "apk" in the command line. If additional phase adjustments are required to further improve the spectrum, click on the "Process tab," then click on the "Adjust Phase" icon to enter the phase correction mode.
- Use the zero-order (0) and first-order (1) phase correction icons by dragging the mouse until all the signals are in negative absorption mode. Apply and save the phase correction values by clicking the "Return and Save" button to exit the phase correction mode.
- Increase the tau until all peaks are either positive or nulled by repeating steps 2.2.2.6-2.2.2.9. To determine the T1 value, simply divide the tau value where the peak is nulled with ln2.
- T1 measurement measured by the null method16 for 13C
- Create a new data set for 13C (see step 2.1.9)
- Type "pulprog t1irpg" to change the pulse sequence to the inversion-recovery experiment for carbon nuclei.
- Type the following commands on the command line to set up the spectral width in ppm, the center of the RF transmitter, the number of scans, the number of dummy scans and the number of data points: "sw 200", "o1p 98", "ns 8", "ds 2"and "td 64K".
- Type "p1 (value)" and enter the duration values for 90° pulse as determined by the pulse calibration (see step 2.2.1) and type "p2 (value)" for the 180° pulse (the duration value is the 90° pulse duration multiplied by two).
- Set the recycle delay to a very large value, such as 100 s by typing "d1 100".
- Set tau to a short value, such as 100 ms by typing "d7 100ms" in the command line.
- Set the receiver gain (RG) to an appropriate value using the command "rga" for automatic calculation of RG.
- Run a spectrum by typing the command "zg".
- Execute Fourier-transformation by typing "efp" in the command line.
- Perform Automatic phase correction by typing the command "apk" in the command line. If additional phase adjustments are required to further improve the spectrum, click on the "Adjust Phase" icon and the phase correction icons for zero-order (0) and first-order phase (1) correction.
- While clicking on the zero-order and first-order phase correction icons, drag the mouse until all the signals are in negative absorption mode. Apply and save the phase correction values by clicking the "Return and Save" button to exit the phase correction mode.
- Increase the tau until all peaks are either positive or nulled by repeating steps 2.2.3.6-2.2.3.9. To determine the T1 value, simply divide the tau value where the peak is nulled with ln2.
- 90° pulse calibration
- One-dimensional (1D) NMR Spectra
- 1H-NMR spectra
- Acquisition of the NMR data
- Go to the 1H data set created in step 2.1.7 and use the standard "pulse-acquire" pulse sequence, "zg", by typing "pulprog zg" in the command line.
- Type the following commands on the command line to set up the spectral width in ppm, the center of the RF transmitter, the number of scans, the number of dummy scans, the number of data points and the pulse duration for a 90° pulse angle: "sw 8", "o1p 3.8", "ns 2", "ds 2", "td 64K" and "p1 (as determined by pulse calibration)" (see step 2.2.1).
NOTE: 32K data points can be used for the 500 MHz instrument. - Set a relaxation delay of 7 s for the 500 MHz instrument or 9 s for the 850 MHz instrument by typing "d1 7s" or "d1 9s", respectively, in the command line.
- Set the receiver gain (RG) to an appropriate value using the command "rga" for automatic calculation of RG.
- Type "digmod baseopt" to acquire a spectrum with improved baseline.
- Start the acquisition by typing the pulse-acquire command "zg" in the command line.
- Processing of the NMR data
- Type "si 64K" in the command line to apply zero-filling and set the size of the real spectrum to 64K.
- Set the line broadening parameter to 0.3 Hz by typing "lb 0.3" in the command line to apply a weighting function (exponential decay) with a line broadening factor of 0.3 Hz prior to Fourier transform.
- Execute Fourier-transformation by typing "efp" in the command line.
- Perform Automatic phase correction by typing the command "apk" in the command line. If additional phase adjustments are required to further improve the spectrum, click on the "Process tab," then click on the "Adjust Phase" icon and the phase correction icons for zero-order (0) and first-order (1) phase correction.
- While clicking on the zero-order and first-order phase correction icons, drag the mouse until all of the signals are in positive absorption mode. Apply and save the phase correction values by clicking the "Return and Save" button to exit the phase correction mode.
- Apply a polynomial fourth-order function for base-line correction upon integration by typing the command "abs n".
NOTE: This ensures a flat spectral baseline with a minimum intensity. - Report chemical shifts in ppm from TMS (δ = 0). Click on the calibration ("Calib. Axis") icon, and place the cursor with the red line on top of the TMS NMR signal (peak closest to 0). Left click and type in "0".
- NMR data analysis
- Integrate the spectral region from δ 1.1 to δ 0.6 as well as the peaks at δ 4.98, δ 5.05 and δ 5.81 using the "Integrate" icon (under the "Process" tab) and the highlight ("Define new Region") icon. Left click and drag through the integrals.
NOTE: If there is need to focus on a region, click on the highlight icon to deactivate and left click and drag the mouse to zoom in on the region. To adjust the threshold intensity, use the middle mouse button if needed. Click on the highlight icon again to make the integration function active, then move to the next peak.- Normalize the sum of the above integrals to 100 by right clicking on the integral value that appears under the signal and select "Normalize sum of integrals". Input the value "100" in the box and click the "Return and Save" to exit the integration mode.
- When using BHT as an internal standard, integrate the peak at δ 6.98 and set the integral equal to the millimoles of BHT per 0.5 mL of the stock solution.
- Integrate the peaks of interest (see step 2.3.1.3.1) extending 10 Hz from each side of the peak, when possible.
- Proceed to perform 13C-NMR spectra acquisition and processing in a similar manner.
- Integrate the spectral region from δ 1.1 to δ 0.6 as well as the peaks at δ 4.98, δ 5.05 and δ 5.81 using the "Integrate" icon (under the "Process" tab) and the highlight ("Define new Region") icon. Left click and drag through the integrals.
- Acquisition of the NMR data
- 13C-NMR spectra
- Acquisition of the NMR data
- Go to the 13C data set and use the inverse gated decoupled pulse sequence, "zgig" by typing "pulprog zgig" in the command line.
NOTE: To run a carbon experiment with the standard broadband decoupled pulse sequence, type "pulprog zgpg" in the command line. - Type the following commands on the command line to set up the spectral width in ppm, the center of the RF transmitter, the number of scans, the number of dummy scans, the number of data points and the pulse duration for a 90° pulse angle: "sw 200", "o1p 95", "ns 16" "ds 2", "td 64K" and "p1 (as determined by pulse calibration)" (see step 2.2.1.4).
- Set a relaxation delay of 35 s for the 500 MHz instrument or 45 s for the 850 MHz instrument by typing "d1 35s" or "d1 45s", respectively, in the command line. When using BHT, relaxation delay should be 50 s in the 500 MHz instrument and 60 s in the 850 MHz instrument.
- Set the receiver gain (RG) to an appropriate value using the command "rga" for automatic calculation of RG.
- Type "digmod baseopt" in the command line to acquire a spectrum with improved baseline.
- Start the acquisition by typing the pulse-acquire command "zg" in the command line.
- Go to the 13C data set and use the inverse gated decoupled pulse sequence, "zgig" by typing "pulprog zgig" in the command line.
- Processing of the NMR data
- Type "si 64K" in the command line to apply zero-filling and set the size of the real spectrum to 64K.
- Set the line broadening parameter to 1.0 Hz by typing "lb 1.0" in the command line to apply a weighting function (exponential decay) with a line broadening factor of 1.0 Hz prior to Fourier transform.
- Execute Fourier-transformation by typing "efp" in the command line.
- Perform Automatic phase correction by typing the command "apk" in the command line. If additional phase adjustments are required to further improve the spectrum, click on the "Process tab," then click on the "Adjust Phase" icon and the phase correction icons for zero-order (0) and first-order phase (1) correction.
- While clicking on the zero-order and first-order phase correction icons, drag the mouse until all of the signals are in positive absorption mode. Apply and save the phase correction values by clicking the "Return and Save" button to exit the phase correction mode.
NOTE: For carbon spectra recorded on Larmor frequency of 214 MHz (the 850 MHz instrument) the correction of the frequency dependent errors (first-order) may be challenging and time consuming for less experienced users because of the large off-resonance effects of the 90° pulse.
- While clicking on the zero-order and first-order phase correction icons, drag the mouse until all of the signals are in positive absorption mode. Apply and save the phase correction values by clicking the "Return and Save" button to exit the phase correction mode.
- Apply a polynomial fourth-order function for base-line correction upon integration by typing the command "abs n" in the command line.
- Report chemical shifts in ppm from TMS (δ = 0). Click on the calibration ("Calib. Axis") icon, and place the cursor with the red line on top of the NMR signal to be referenced. Left click and type in "0".
- NMR data analysis
- Integrate the spectral region from δ 175 to δ 171 using the "Integrate" icon (under the "Process" tab) and the highlight ("Define new Region") icon. Left click and drag through the integrals.
NOTE: If there is need to focus on a region, click on the highlight icon to deactivate and left click and drag the mouse to zoom in on the region. Click on the highlight icon again to make the integration function active, then move to the next peak.- Set the integral to 100 by doing a right click on the integral value that appears under the signal and select "Calibrate Current Integral". Input the value "100" in the box and click the "Return and save" to exit the integration mode.
- When using BHT as an internal standard, integrate the peak at δ 151.45 and set the integral equal to the millimoles of BHT per 0.5 mL of the stock solution.
- Integrate the peaks of interest extending 5 Hz from each side of the peak (see step 2.3.2.3.1).
- Integrate the spectral region from δ 175 to δ 171 using the "Integrate" icon (under the "Process" tab) and the highlight ("Define new Region") icon. Left click and drag through the integrals.
- Acquisition of the NMR data
- 1H-NMR spectra
Representative Results
1H and 13C NMR spectra were collected for commercially available fish oil supplements using two NMR instruments; an 850 MHz and a 500 MHz spectrometer. These spectra can be used for the quantitative determination of components of fish oil, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), as well other compounds such as n-1 acyl chains and nutritionally important index such as the n-6/n-3 ratio. The quantification can be performed even without the use of an internal standard, however, the quantitative results must be expressed as relative molar percentages. When the data need to be expressed in absolute values (mg/g), an internal standard is required. The results obtained by NMR are highly reproducible with relative standard deviations (RSD) ranging from 0.3% to 2% for 13C NMR analysis and from 0.5% to 2.5% for 1H NMR analysis, depending on the lipid. The slightly higher RSD for 1H NMR is often observed because proton spectra tend to be overcrowded, which affects the accuracy of the analysis, especially for resonances that have a lower signal to noise ratio (S/N). A very good agreement was found between the 850 MHz and the 500 MHz instrument with RSDs ranging from 1% to 4%. Relatively high RSDs (up to 8%) were observed when comparing results obtained by 1H and 13C, especially for compounds that appear in lower concentrations such as n-1 acyl chains. NMR spectroscopy has been previously validated as a tool for lipid analysis, including the determination of some fish oil components. Results showed that it is in good agreement with traditional methods, such as GC17,18.
1H NMR Analysis
Figure 1 compares the 1H NMR spectra acquired on (A) an 850 MHz and (B) a 500 MHz instrument. The 850 MHz spectrum is characterized by higher resolution, however the major components of fish oil including DHA, EPA, and n-6/n-3 ratio can also be determined from the 500 MHz spectrum. The 1H-NMR signals of fish oil fatty acids that can be used for quantitation purposes are shown in Table 1, whereas the complete NMR assignment of the 1H NMR spectrum of fish oil can be found elsewhere19.
1H NMR gave reliable data for the quantification of the total amount of n-3, n-6, DHA, trans fatty acids, n-1 acyl chains, and saturated fatty acids (SFA). For the 1H NMR analysis, the use of appropriate relationships is required because most of the signals belong to groups of protons that are common to different fatty acids and lipids. For that reason, in most cases the concentration of fatty acids in fish oil can be determined only by combination of various 1H NMR signals, incorporated in the appropriate relationships. In addition, these equations contain arithmetic coefficients that normalize the different number of protons associated with each group. When an internal standard is used the following equation should be considered: C = I/IIS × NIS/N × A × MW/m (1), where C is the concentration of the analyte in mg/g of fish oil, I is the integral of a resonance that is uniquely attributed to the lipid of interest, IIS is the area of a proton signal that belongs uniquely to the internal standard, N is the number of protons of the functional group that is analyzed, NIS is the number of protons of the internal standard that are used for the analysis, A is the millimoles of internal standard, MW is the molecular weight of the fatty acid (expressed in methyl esters), and m is the amount of fish oil expressed in g.
Example 1, DHA: The proportion of DHA is determined by the equation CDHA = ¾ IDHA/S, where IDHA is the integral of the signal at δ 2.39 which belongs to the Hα and Hβ protons of DHA, and S is the sum of integrals of the methyl protons of SFA, n-6, n-9, n-3, trans fatty acids plus the integrals of the peaks of n-1 acyl chains at δ 4.98, δ 5.05 and δ 5.81. The integral IDHA is normalized by multiplying by 3/4 because it corresponds to four protons, whereas the integral S corresponds to three protons. 1H NMR is not capable to giving information about positional distribution of fatty acids on the glycerol backbone and thus can only be used for the quantification of the total amount of fatty acids. The 1H NMR analysis of an encapsulated fish oil supplement showed that it consists of 10.5% of DHA. The concentration of DHA in the same sample using BHT was found to be 105.23 mg/g. These values are very close to the values obtained with 13C NMR (see example 2 for 13C analysis).
Example 2, n-1 acyl chains: The concentration of n-1 acyl chains is given by the relationship Cn-1 = 3In-1/S, where In-1 is the integral of the signal at δ 5.818. This signal corresponds to one proton and thus needs to be normalized by multiplying by three. When using BHT, n-1 acyl chains are determined by the equation Cn-1 = 2In-1/IBHT. The results cannot be expressed in mg/g because the MW of n-1 acyl chains is unknown.
Example 3, n-6/n-3 ratio: This important index can be calculated from the ratio of the normalized intensities of the resonance at δ 2.77, which corresponds to the bis-allylic protons of n-6 acyl chains (two protons) over the triplet at δ 0.97 that belongs to n-3 fatty acids and corresponds to three protons. The relationship is Cn-6/ Cn-3 = 3/2 IA/IB, where IA and IB are the integrals of the signals at δ 2.77 and δ 0.97, respectively. n-6 fatty acids are determined from the relationship Cn-6 = 3/2In-6/S, where In-6 is integral of the bis-allylic protons at δ 2.77.
Example 4, trans fatty acids: Trans fatty acids can be calculated from the equation Ctrans= Itrans/S, where Itrans is the integral of the signal at δ 0.91. The present sample contained 3.07% of trans fatty acids, as determined by 1H NMR using the 850 MHz instrument. The same sample analyzed in a 500 MHz instrument was found to contain 3.03% of trans fatty acids.
Example 5, saturated fatty acids (SFA): The concentration of SFA can be calculated from the equation CSFA = S–Cn-3 –Cn-6 –Cn-9 –Cn-1 – Ctrans. n-9 fatty acids (mainly oleic acid) can be quantified according to the equation Cn-9 = (3/4Q – 3/2In-6)/S, where Q is the integral of the allylic protons of n-6 and n-9 at δ 2.01. The amount of SFA in a commercially available fish oil sample was found to be 36.1%. The same sample analyzed with 13C NMR was found to contain 33.8% SFA. SFA represent a group of various FA (e.g. stearic and palmitic) with different MW and thus their concentration if fish oil cannot be expressed in mg/g.
Example 6, total sterols: The amount of total sterols (free and esterified) can be determined by the signal of the methyl protons at carbon 18 which appears at δ 0.68, using the equation C = Iste/S. The molar ratio of total sterols in a commercially available fish oil sample was found to be 0.32%. BHT can also be used for the determination of the absolute concentration of sterols. The main sterols in fish oil are cholesterol and vitamin D (or its precursor 7-dehydrocholesterol) and are often added in the supplements. These compounds have a very similar MW. Therefore, results can be expressed in mg/g and are calculated according to the equation C = 2/3 ISTE/IISA × MWSTE/m, where MWSTE is the molecular mass (386) of cholesterol, which constitutes the majority of the sterolic fraction in fish oil20. The amount of sterols in the same sample using BHT was 3.8 mg/g of fish oil. The individual determination of cholesterol (δ 0.680) and 7-dehydrocholesterol (δ 0.678) is feasible on an 850 MHz instrument after the application of a window function for resolution enhancement.
13C NMR Analysis
Figure 2 illustrates the 13C NMR spectra acquired on (A) an 850 MHz and (B) a 500 MHz instrument in the carbonyl carbon area. The two spectra are very similar and can provide the same amount of information. The 13C NMR spectrum can be successfully used for the analysis of additional fatty acids such as stearidonic (SDA) and eicosatetraenoic (ETA) acids, however more scans are required for samples in which these acids are in lower concentrations. The 13C spectra are characterized by high resolution because of the large spectral width and the application of broadband decoupling, which eliminates the effect of scalar coupling and produces singlets. For this reason, there is limited overlapping even when using a 500 MHz instrument.
The 13C NMR spectrum is much more informative compared to the 1H NMR spectrum and can provide more comprehensive quantitative data because less signal overlapping is observed (Figures 1 and 2). The most useful spectral region of the 13C spectrum is the carbonyl carbon region because it provides quantitative information for a large number of fatty acids as well as for their positional distribution on the glycerol skeleton19,21,22. The methyl group area from δ 14.5 to δ 13.5 can be used for the quick determination of the total amount of n-3, n-6, n-9 and saturated fatty acids (SFA), as well as trans fatty acids. However, in the 500 MHz NMR spectrometer, there is a partial overlapping of the n-6 and n-9 saturated fatty acids (SFA). The application of a window function for resolution enhancement may solve this problem although the 850 MHz instrument is still considered a more reliable option. The olefinic region of the carbon spectrum can be used for the total amount of n-3 and n-1 acyl chains as well as for determination of individual fatty acids such as DHA, EPA, Arachidonic acid (AA), Linolenic (Ln) n-3, and oleic acid (OL) (see Table 2). 13C NMR can be also applied for the characterization of fish oil from other sources, such as supplements rich in ethyl esters (EE) using the carbon signals at δ 14.31 (methyl) and δ 60.20 (methylene).
For carbon analysis, fatty acids can be determined by dividing the integral of the appropriate aliphatic, olefinic, and carbonyl signals with the total integral of all acyl chains, according to the general relationship C = I/S (2), where C is the concentration of the analyte in mole (%), I is the integral of a resonance that is uniquely attributed to the lipid of interest, and S is the total integral of signal(s) that represents the total lipid content of the sample. The total integral S of acyl chains can be determined by integrating the region from δ 175 to δ 171 and is set to 100.
Quantification of fatty acids in mg/g of fish oil is performed using an internal standard on the basis of the following relationship: C = I/IIS × A× MW/m (3), where C is the concentration of the analyte in mg/g of fish oil, I is the integral of a resonance that is uniquely attributed to the lipid of interest, IIS is the area of a carbon signal that belongs uniquely to the internal standard, A is the millimoles of internal standard, MW is the molecular weight of the compound of interest (for fatty acids expressed in methyl esters), and m is the amount of fish oil in g. The 13C-NMR signals of fish oil fatty acids that can be used for quantitation purposes are shown in Table 2, whereas the complete NMR assignment of the 13C NMR spectrum can be found elsewhere19.
Example 1, EPA at sn-2 position: The amount (%) of EPA on the sn-2 position is calculated by dividing the integral of the signal at δ 172.56 by S. The amount of EPA at the sn-2 position in a commercially available sample was found to be 3.4% using the 850 MHz instrument. Using the same spectrometer and BHT as an internal standard, the amount of EPA at the sn-2 position expressed in mg/g of fish oil is 29.73 mg/g. The same sample analyzed in a 500 MHz instrument was found to contain 3.6% or 31.39 mg/g of EPA in the sn-2 position. Similar results can be obtained when calculating the relative molecular ratios of EPA at sn-2 using a fully decoupled spectrum. This is because the carbonyl carbon of EPA is affected by proton decoupling to the same negligible degree as the other carbonyl carbons, which are used as reference. However, large deviations are observed when using BHT, because the carbon of BHT at δ 151.45, which is used for quantification, receive a different NOE enhancement compared to the carbonyl carbons of fatty acids. For that reason, the fully decoupled spectrum should be avoided when using internal standards or integrating carbons with different multiplicities.
Example 2, total amount of DHA: The total amount (%) of DHA is simply calculated by adding the amounts of DHA in sn-1,3 and sn-2 position as determined by the NMR signals at δ 172.48 and δ 172.08, respectively. The same sample analyzed with 1H NMR (see example 1 of 1H analysis) was found to contain 10.3% DHA according to 13C NMR analysis. The amount of DHA can also be expressed in mg/g by using an internal standard and Equation 3. The total amount of DHA was 103.25 mg/g.
Example 3, total amount of SDA: The total amount (%) of SDA is determined by adding the integrals of the signals at δ 172.99 and δ 172.60 which belong to the carbonyl carbons of SDA on position sn-1,3 and sn-2, respectively, then dividing the sum by S. The sample analyzed was found to contain 3.93% SDA or 34.54 mg/g.
Example 4, n-3 Ln: n-3 Ln (%) can be determined by dividing the integral of the signal at δ 131.85 with the integral S. The molar ratio of n-3 Ln in the analyzed fish oil sample was 0.7%. The absolute concentration using BHT was calculated as 5.5 mg/g.
Example 5, trans fatty acids: The molar ratio of trans fatty acids is determined by dividing the integral of the signal at δ 13.80 with S. The analysis of the same sample that was analyzed with 1H NMR and was found to be 3.07% of trans FA, was also analyzed with 13C NMR and its trans fatty acid content was found to be 3.42%. The 13C NMR analysis of the same sample on a 500 MHz instrument showed a 3.64% content of trans fatty acids. The amount of trans FA in mmol/g of fish oil can be determined using BHT as an internal standard and the equation C = I/IIS × A/m, however results cannot be expressed in mg/g because the peak at δ 13.80 corresponds to various trans fatty acids, mainly trans DHA and trans EPA, with different MW.
Example 6, EE: The concentration of EE in a fish oil sample is calculated by dividing the integral of the spectral area from δ 60.50 to δ 60.00, which corresponds to the methylene carbons of the EE of various fatty acids, with S. The analysis of an EE fish oil sample showed that it consisted of 100% EE. It should be noted that in EE samples, EPA can be calculated either by the carbonyl peak at δ 173.60 or by the methylene EE carbon at δ 60.20, whereas DHA can be calculated using the signal at δ 60.31 and/or the signal at δ 173.09.
A complete list of the diagnostic signals that can be used for quantification purposes with 13C and 1H NMR analysis can be found in Tables 1 and 2, respectively, whereas a detailed description of the equations that can be used for this analysis can be found elsewhere19.
NMR can additionally be applied for the assessment of the oxidation status of fish oil supplements. Figure 3 compares the 1H NMR spectra of a fish oil sample under two oxidation conditions; exposure to heating and exposure to ultraviolet (UV) light. Lipid oxidation is a complicated process, and the composition of oxidation products depends on the conditions of oxidation. The main oxidation products are hydroperoxides (δ 8.0-8.8), conjugated dienes hydroperoxides (δ 5.4-6.7), and aldehydes (δ 9.0- 10).
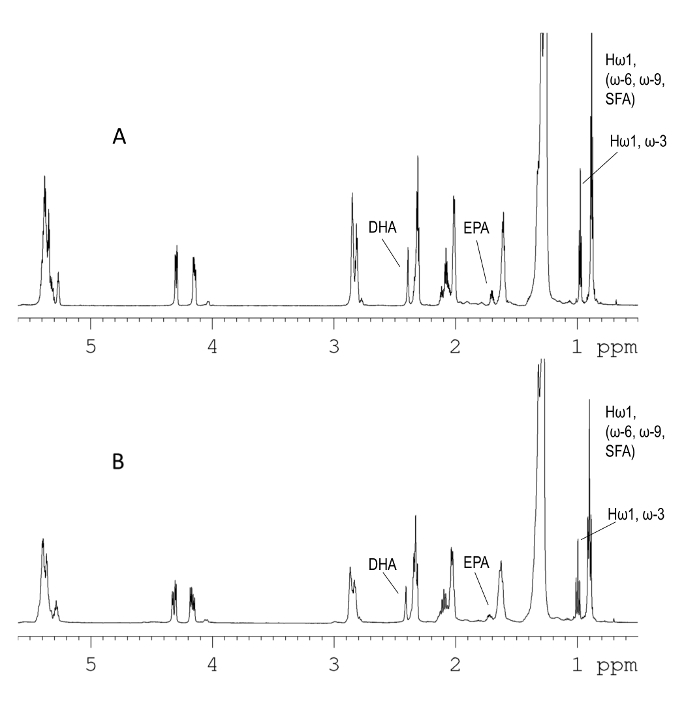
Figure 1. The 1H NMR analysis. 850.23 (A) and 500.20 MHz (B) 1H-NMR spectrum of a fish oil supplement in CDCl3 solution. The NMR signals of EPA and DHA that can be used for their determination are shown. The peak at δ 0.97 can be used for the determination of the total amount of n-3 fatty acids. The envelop at δ 1.39-1.20 is cropped, as it belongs to the methylene protons of all fatty chains and cannot be used for any identification or quantification purposes. The 1H NMR spectrum is characterized by a narrower spectral width (SW) compared to the 13C NMR spectrum and thus by lower spectral resolution. Please click here to view a larger version of this figure.
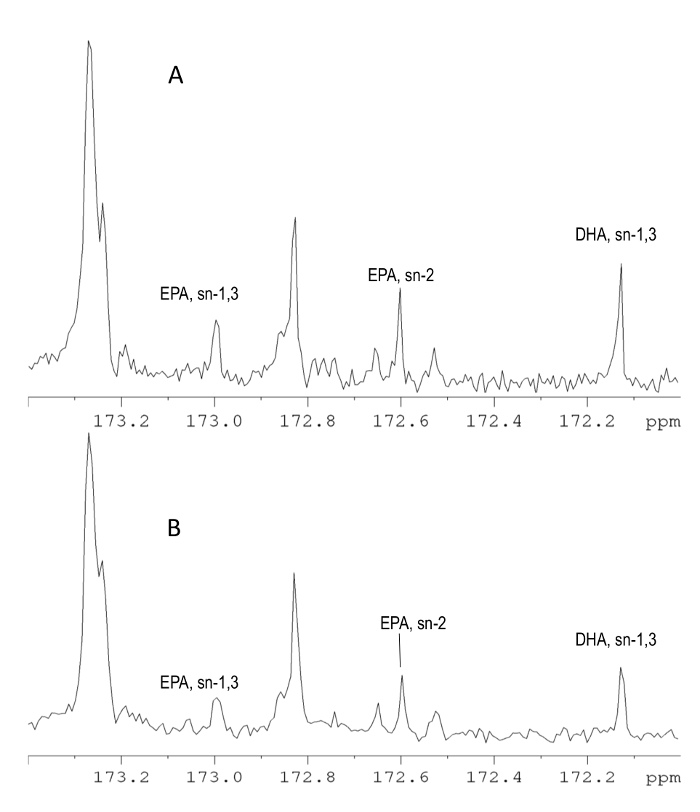
Figure 2. The 13C NMR analysis. 213.81 (A) and 125.77 MHz (B) 13C-NMR spectrum of a fish oil supplement in CDCl3 solution in the carbonyl carbon region. The NMR signals of EPA and DHA on sn-1,3 and sn-2 position are shown. These signals can be used for the quantitative determination of EPA and DHA. Although the spectra recorded at 213.81 MHz are characterized by a higher resolution and sensitivity, the 125.77 MHz spectra can also be used for the determination of the major compounds. The application of decoupling in the 13C NMR experiment eliminates the effect of scalar coupling between the carbon and hydrogen nuclei and thus the signals appear as singlets making the analysis easier compared to the 1H NMR spectrum. Please click here to view a larger version of this figure.
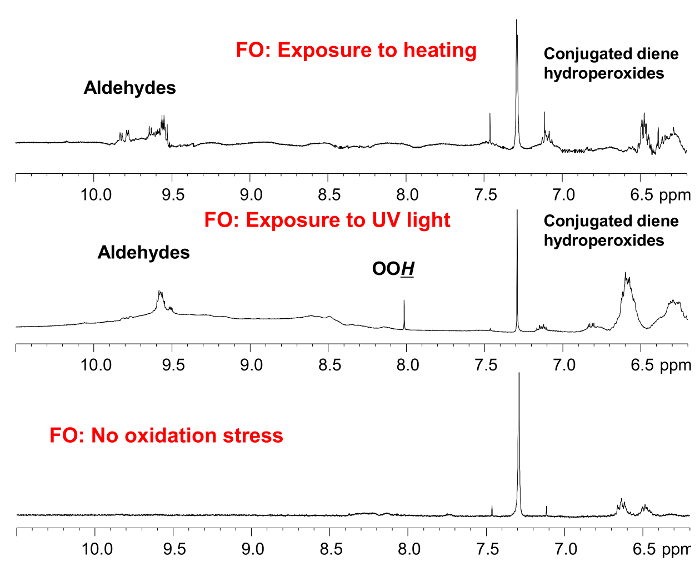
Figure 3. Fish oil oxidation. The 1H NMR spectrum of oxidized fish oil depends on the oxidation conditions. The resonances attributed to hydroperoxides (δ 8.0-8.8), conjugated dienes hydroperoxides (δ 5.4-6.7), and aldehydes are shown. Please click here to view a larger version of this figure.
| δ ppm | Proton | Compound |
| 0.677 | CH3 (18) | Cholesterol |
| 0.678 | CH3 (18) | 7-dehydrocholesterol |
| 0.88 | CH2CH3 (t), Jω1, ω2 = 7.27 Hz | n-9, SFA acyl chains |
| 0.883 | CH2CH3 (t), Jω1, ω2 = 7.08 Hz | n-6 acyl chains |
| 0.911 | CH2CH3 (t), Jω1, ω2 = 7.65 Hz | Trans acyl chains |
| 0.973 | CH2CH3 (t), Jω1, ω2 = 7.63 Hz | n-3 acyl chains |
| 1.25 | CH2CH3 (t), J = 7.20 Hz | Ethyl esters |
| 1.697 | OCOCH2CH2 (t), JHα, Ηβ = Hz | EPA acyl chain |
| 2.391 | OCOCH2CH2 (t) | DHA acyl chain |
| 2.772 | CH=CHCH2CH=CH | n-6 acyl chains |
| 2.81 | CH=CHCH2CH=CH | n-3 acyl chains |
| 3.593 | 3’a-CH2OCO | Glycerol of 1-MAG |
| 3.722 | 3’a, 3’b-CH2OCO (br) | Glycerol of 1,2-DAG |
| 4.073 | 2’-CHOH (br) | Glycerol of 1,3-DAG |
| 4.121 | CH2CH3 multiplet | Ethyl esters |
| 4.173 | 1’b, 3’b-CH2OCO (dd) | Glycerol of 1,3-DAG |
| 4.238 | 1’a-CH2OCO (dd) | Glycerol of 1,2-DAG |
| 4.329 | 1’b-CH2OCO (dd) | Glycerol of 1,2-DAG |
| 4.989 | -CH=CH2 cis (dd) | n-1 acyl chains |
| 5.052 | -CH=CH2 trans (dd) | n-1 acyl chains |
| 5.082 | 2’-CHOCO | Glycerol of 1,2-DAG |
| 5.268 | 2’-CHOCO | Glycerol of TAG |
| 5.436 | CH=CHCH2CH=CH2 | n-1 acyl chains |
| 5.818 | -CH=CH2 | n-1 acyl chains |
Table 1: The assignment of the 1H NMR spectrum. The 1H-NMR chemical shifts of fish oil fatty acid signals that can be used for quantification purposes in CDCl3 solution are presented. The chemical shifts are measured in ppm and provide information about the chemical environment of the nuclei.
| δ ppm | Carbon |
| 173.24 | C1 SFA (sn-1,3) |
| 172.21 | C1 OL, LO (sn-1,3) |
| 173.16 | C1 ETA (sn-1,3) |
| 173.13 | C1 DPA (sn-1,3) |
| 173.03 | C1 SDA (sn-1,3) |
| 172.97 | C1 EPA (sn-1,3) |
| 172.73 | C1 ETA (sn-2) |
| 172.69 | C1 DPA (sn-2) |
| 172.61 | C1 SDA (sn-2) |
| 172.56 | C1 EPA (sn-2) |
| 172.48 | C1 DHA (sn-1,3) |
| 172.08 | C1 DHA (sn-2) |
| 136.8 | Cω1, n-1 |
| 131.85 | Cω3 LN |
| 130.37 | C15 AA |
| 130.11 | C9 LN |
| 130.06 | C13 LO |
| 129.54 | C5 DHA sn-2 |
| 129.47 | C5 DHA sn-1,3 |
| 128.94 | C5 EPA |
| 128.76 | C6 EPA |
| 128.45 | C17 n-3 |
| 127.71 | n-3 |
| 127.53 | C4 DHA sn-2 |
| 127.5 | C4 DHA sn-1,3 |
| 126.86 | Cω4, all n-3 |
| 114.71 | Cω2, n-1 |
| 60.08 | DHA, Ethyl esters |
| 59.96 | EPA, Ethyl esters |
| 59.95-59.85 | Other FA, Ethyl esters |
| 33.48 | C2 EPA sn-2 |
| 33.32 | C2 EPA sn-1,3 |
| 31.44 | C3 n-1 |
| 27.05 | Allylic n-6 |
| 26.49 | C4 EPA sn-1,3 |
| 26.47 | C4 EPA sn-2 |
| 24.6 | C3 EPA |
| 24.48 | C3 SDA sn-1,3 |
| 24.44 | C3 SDA sn-2 |
| 14.27 | Cω1, all n-3 |
| 14.13 | Cω1, SFA |
| 14.11 | Cω1, OL |
| 14.07 | Cω1, LO |
| 13.8 | Cω1, trans FA |
Table 2: The assignment of the 13C NMR spectrum. The 13C-NMR chemical shifts of fish oil fatty acid signals that can be used for quantitation purposes in CDCl3 solution are presented.
Supplemental Figure S1: Comparison between the 13C NMR spectra acquired using the standard broadband decoupling (A) and the inverse gated decoupling (B) pulse sequences. The spectra were recorded for the same sample with the same number of scans, processed with the same processing parameters and are shown with the same scale factor. Please click here to download this figure.
Discussion
Modifications and Strategies for Troubleshooting
Spectral quality. The linewidth of the NMR signal and thus the resolution of the NMR spectrum is highly dependent on shimming, which is a process for the optimization of the homogeneity of the magnetic field. For routine analysis, 1D shimming is adequate and a 3D shimming is not required, given that it is performed by NMR personnel on a regular basis. If this is not the case, a 3D shimming must be performed prior to analysis using a sample containing 0.6 mL of H2O:D2O (90:10). To achieve a better and faster shimming, the sample needs to be centered in the excitation/detection region of the radio frequency (RF) coil, using the graded depth gauge, before it is placed in the magnet bore. Another factor that affects shimming is spinning the sample at a spin rate of 10-20 Hz. Although spinning the sample at this spin rate improves the radial shims (X, Y, XY, XZ, YZ, X2-Y2, etc.), it is generally not recommended in order to avoid the appearance of spinning side bands of the first or higher order. However, when working on instruments that operate in Larmor frequencies lower than 400 MHz, spinning is recommended for 1D NMR experiments.
Resolution, as well as sensitivity, are affected by the receiver gain (rg) value. Low values of the receiver gain reduce the sensitivity, whereas values higher than appropriate cause overflow of the analog-to-digital converter (ADC). ADC overflow results in nonsymmetrical line-shapes and the signals cannot be used for quantitative purposes because the first points of the free induction decay (FID) may be lost. In most cases, the command "rga" calculates an appropriate rg value. However, in some cases, the rg value calculated by the software is higher than the ideal value and there is a distortion in the Lorentzian shape of the NMR signal. In such a case, the user should manually input a smaller rg value by typing "rg (value)" in the command line. A typical RG value for the samples that analyzed with this protocol is 8.
Often, when using cryogenically cooled NMR probes with a high Quality-factor (Q-factor), a large delay (dead time) >200us between the last pulse and the detection period is required to avoid artifacts such as a hump around the transmitter's frequency and a rolling in the spectrum's baseline. However, such a long delay causes a large negative first-order phase error, which can also introduce a baseline rolling and large dips around the base of the strong signals. In these cases, a z-restored spin-echo pulse sequence can be used to produce NMR spectra with significantly improved baselines, although a small sensitivity reduction may occur23.
Phospholipids. In addition to the analysis of fish oil samples rich in triglycerides and ethyl esters, NMR can be used for the analysis of fish oil samples rich in phospholipids (PLs). However, special care is required for such samples because PLs form aggregates, which can cause a significant reduction in the spectral resolution and sensitivity. For the analysis of these samples, a solvent mixture of deuterated chloroform:methanol (CDCl3:CD3OD) in a ratio of 70:30 is required for obtaining spectra of high quality.
Internal standard. BHT was selected as an internal standard in this study because it is a highly symmetric molecule with simple 1H and 13C NMR spectra and none of its peaks overlap with those of fish oil constituents. BHT has a signal in the 1H NMR spectrum, which appears as a singlet at δ 6.97 and belongs to the two equivalent aromatic protons (para– position in respect to the OH group) and a signal at δ 151.45 in the 13C NMR spectrum which belongs to the aromatic quaternary carbon bearing the -OH group. Both of these signals have no overlap with any of the constituents in fish oil, and thus can be used for quantification purposes. Other compounds such as 1,2,4,5-tetrachloro-3-nitrobenzene (TCNB) or ethylene chloride can also be used as alternative internal standards, however, they are characterized by longer T1 values.
Limitations of the Technique
The quantification of various fatty acids and lipids in fish oil supplements is achieved through integration of the appropriate diagnostic NMR signals in the 1D spectra. Such signals should belong only to a specific sample component and must have no interference with signals from other compounds. This may be an issue for 1H NMR analysis since the 1H NMR spectrum is characterized by low resolution due to the short range of chemical shifts. In addition, the presence of scalar coupling (J) produces multiplets and makes the analysis more complicated. For example, ethyl esters (EE) can be quantified using 1H NMR by the characteristic triplet (J = 7.20 Hz) of the methyl group at δ 1.25 and the multiplet at δ 4.12, which belongs to the methylene protons of the ester group. However, when using NMR instruments operating in Larmor frequencies lower than 850 MHz, the analysis of EE using 1H NMR should be avoided because of the partial overlapping of the peak at δ 4.12 with the peak at δ 4.14 of TGs, and the overlapping of the signal at δ 1.25 with the broad signal of the aliphatic methylene protons at δ 1.23-1.35. Large deviations were also observed between the 1H and 13C analysis of EPA in some samples, 13C NMR was closer to the labeled composition provided by the manufacturer. This is probably due to the overlapping of the signal at δ 1.69, which is used for EPA analysis, with signals of other compounds that appear in some types of fish oil supplements. Additional errors in quantifications can arise when using an internal standard due to the uncertain purity of the internal standard and from errors in weighing.
The compositional analysis can be expressed in relative molar concentrations without the use of an internal standard. If results need to be expressed in absolute concentrations, for example as milligram of fatty acid per gram of oil (mg/g), the use of an internal standard is required. However, in cases where the NMR signal of interest belongs to multiple compounds with different molecular weights, the results cannot be expressed as mg/g even when using an internal standard. In addition, the use of internal standard usually increases the length of the analysis because the most common internal standards, such as BHT, are small molecules with high molecular symmetry, which results in long relaxation times. Since the repetition time (delay between pulses + acquisition time) is set according to the longest relaxation time T1 in the sample, the use of an internal standard will increase the duration of the experiments as longer delays between pulses are required. This is an especially important factor for 13C NMR analysis because of the exceptionally long T1 relaxation time of carbon nuclei. The addition of a paramagnetic compound such as Cr(acac)3 can efficiently reduce the T1 relaxation time. The recommended concentration of Cr(acac)3 is 0.75 mg/mL of solution. Higher concentrations of Cr(acac)3 may be considered for further reduction of T1, however, caution is required in order to avoid decreases in the S/N due to the line broadening.
Although the 13C NMR is characterized by a much higher spectral resolution compared to 1H, the sensitivity of the 13C NMR experiment is significantly lower because of the low natural abundance (1.1%) and the low gyromagnetic ratio (67.26 106 rad s-1 T-1) of 13C nuclei. In addition, the long T1 relaxation times of 13C increase the length of the analysis. This may be an issue when the available oil for analysis is limited, because an increased number of scans should be used to achieve a reasonable signal to noise ratio.
Limitations in the sensitivity and the resolution of the NMR spectra prevent the analysis of many minor compounds in fish oil that can be analyzed with other techniques such as GC. For example, 1Η NMR is unable to separate individual sterols or fatty acids (e.g. palmitic and stearic) whereas 13C is not able to determine compounds that appear in very low concentration in fish oil such as dodecanoic and myristic acid, which overlap with the signals of all saturated fatty acids at δ 173.24 and δ 172.82. Although increasing the amount of sample that is analyzed makes the analysis of some minor compounds feasible, caution is required for very concentrated samples, because of their increased viscosity. Very viscous solutions containing more than 150 mg of oil should be avoided because there is a decrease in S/N due to the line broadening caused by the reduced spin-spin T2 relaxation times. In addition, longer delays between pulses are required because of the longer T1 and there are several issues in shimming and thus in resolution.
All the compounds analyzed in fish oil with NMR can be quantified simultaneously in one snapshot without using any separation or purification steps. The NMR analysis is rapid as the 1H spectrum can be recorded in less than one minute, whereas the 13C NMR acquisition lasts 10 min. It should be noted, however, that there are a few factors that affect the data acquisition time. Specifically, for 13C NMR, the 10 min run time can only be achieved without the use of internal standards, and with the use of cryogenically cooled probes, in which the RF coil and the preamplifier are cooled and thus the thermal noise is minimized. A 10-15 fold increase in the experimental time should be expected for 13C NMR analysis when room temperature (conventional) probes are used.
Significance with Respect to Existing Methods
NMR spectroscopy proved to be a powerful tool for qualitative and quantitative determination of the composition of fish oil supplements, and because of its rapidness it has the potential to be applied for the high throughput screening of a vast number of fish oil samples. NMR spectroscopy is by definition a quantitative methodology since the signal area is directly proportional to the number of nuclei that cause the signal. While acutely toxic chemicals are required to prepare NMR samples, this method is environmentally friendly because such small amounts of this chemicals (e.g. CDCl3) are used as opposed to other methods that require large amounts of solvent to elute samples. In addition, NMR has several advantages compared to other analytical methods. No calibration with standards is required prior the analysis, and a minimal sample preparation without any separation and purification steps is usually adopted, which renders NMR a very fast analytical tool. Additionally, 13C NMR is the best available methodology for determining the positional distribution of various fatty acids on the glycerol skeleton. While the enzymatic hydrolysis has been used as an alternative is not always reliable24. This is of specific importance because there is a significant interest in studying the regiospecificity of various fatty acids in foods, as it has been found that this affects their function in human diet25,26.
Future Applications
Despite the agreement between NMR analysis and the products' label, as well as the fact that there are some studies showing agreement between GC and NMR, we believe that more rigorous and comprehensive intra-laboratory studies are required to examine the agreement between NMR and traditional methodologies for the analysis of fish oil constituents using a larger number of samples, fish oil products of different origins, and certified standard solutions.
Another important future application of NMR in fish oil analysis will be the determination of the oxidation products. In addition to the determination of the major compounds in fish oil, several primary and secondary oxidation products in fish oil, such as aldehydes and peroxides, are present. 1H NMR can be potentially applied for the evaluation of the oxidation status in fish oil supplements, under different oxidation conditions, as shown in Figure 3. The biggest challenge in this analysis will be the NMR assignment and the identification of individual oxidation products. Advances in sensitivity of the NMR hardware will also allow the identification of individual sterols using 13C NMR. NMR spectroscopy can also be applied for the analysis of fish tissue as a whole even without any extraction by using High Resolution Magic Angle Spinning (HR-MAS) NMR.
Critical Steps within the Protocol
Two of the most critical steps that affect the accuracy of quantitative NMR spectra involve the selection of a 90° pulse and the use of a delay between pulses ≥ 5×T1. The pulse angle is proportional to pulse width which is a calibrated NMR parameter that depends on the instrumentation and the sample. A 90° pulse is essential for the complete conversion of longitudinal (z) magnetization to the observable transverse (xy) magnetization. It is important to note that before pulse calibration, the NMR probed needs to be well tuned and matched. This will optimize the transfer of the RF power to the sample and thus maximize S/N and ensure effective decoupling. The probe tuning is mostly affected by the dielectric constant of the sample, so if there are differences in the concentration between samples, repeat the tuning process for each one. The 1D 13C NMR experiment involves both 13C and 1H channels so automatic tuning and matching is necessary for both nuclei.
A delay between pulses longer than 5×T1 ensures the complete recovery of the net magnetization to its initial value. If all the resonances in the spectrum have not completely relaxed before each pulse, the signal is partially suppressed and this leads to inaccuracies in the integration. T1 value is a critical factor that affects the length of the experiment and it depends on the magnetic field strength as well as the viscosity of the sample. Given that the viscosity between samples is similar, T1 relaxation times should be determined for each instrument only in the beginning of the analysis session.
Another important feature of the fish oil analysis with 13C NMR is the selection of the appropriate pulse sequence. The most reliable method for quantitative 13C analysis is the inverse gated decoupling experiment, where broadband proton decoupling is applied only during the acquisition period and thus there is no polarization transfer from 1H to 13C via the nuclear Overhauser effect (NOE). However, while the fully decoupled NMR experiment can be used for quantitative purposes, caution is required when using this experiment because there are different NOE factors among carbons with different multiplicities and therefore integral comparison between methyl, methylene, methane and carbonyl carbons must be avoided. Despite this, when only carbons of similar multiplicity and chemical environment are considered in the analysis, the fully decoupled method is reliable. One example of this is carbonyl carbons of fatty acids which have been found to have no significant differences in the NOE factors after decoupling27. In addition, for carbons bearing protons, the fully decoupled experiment provides higher sensitivity due to the NOE contributions on the NMR signal intensity. A comparison between spectra acquired with the two pulse sequences is shown in Figure S1.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Foods for Health Discovery Theme at The Ohio State University and the Department of Food Science and Technology at The Ohio State University. The authors would like to thank the NMR facility at The Ohio State University and the NMR facility at Penn State University.
Materials
| Avance III 850 NMR instrument | Bruker | ||
| Avance III 500 NMR instrument | Bruker | ||
| TCI 5mm probe | Bruker | Helium cooled inverse (proton deetected) NMR probe featuring three independent channels (1H, 13C, 15N) | |
| BBO prodigy 5mm probe | Bruker | Nitrogen cooled observe (X-nuclei detected) probe, featuring two channels; one for 1H and 19F detectionand one for X-nuclei (covering from 15N to 31P) | |
| Spinner turbin | Bruker | NMR spinners are made by polymer materials and they have a rubber o-ring to hold the NMR tube securely in place | |
| Topspin 3.5 | Bruker | ||
| deuterated chloroform | Sigma-Aldrich | 865-49-6 | 99.8 atom % D, contains 0.03 TMS |
| 2,6-Di-tert-butyl-4-methylphenol, | Sigma-Aldrich | 128-37-0 | purity >99% |
| Fish oil samples | |||
| NMR tubes | New Era | NE-RG5-7 | 5mm OD Routine “R” Series NMR Sample Tube |
| BSMS | Bruker | Bruker Systems Management System; control system device |
References
- Simopoulos, A. P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 56 (8), 365-379 (2002).
- Goodnight, S. H., Harris, W. S., Connor, W. E. The effects of dietary omega 3 fatty acids on platelet composition and function in man: a prospective, controlled study. Blood. 58 (5), 880-885 (1981).
- Harper, C., Jacobsen, T. Usefulness of omega-3 fatty acids and the prevention of coronary heart disease. Am. J. Cardiol. 96 (11), 1521-1529 (2005).
- Kremer, J. M., et al. Effects of high-dose fish oil on rheumatoid arthritis after stopping nonsteroidal antiinflammatory drugs. Clinical and immune correlates. Arthritis and Rheumatol. 38 (8), 1107-1114 (1995).
- Malasanos, T., Stackpoole, P. Biological effects of omega-3 fatty acids in diabetes mellitus. Diabetes Care. 14, 1160-1179 (1991).
- Han, Y., Wen, Q., Chen, Z., Li, P. Review of Methods Used for Microalgal Lipid-Content Analysis. Energ. Procedia. 12, 944-950 (2011).
- Guillén, M., Ruiz, A. 1H nuclear magnetic resonance as a fast tool for determining the composition of acyl chains in acylglycerol mixtures. Eur. J. Lipid Sci. Technol. 105, 502-507 (2003).
- Sacchi, R., Medina, I., Aubourg, S. P., Addeo, F., Paolillo, L. Proton nuclear magnetic resonance rapid and structure specific determination of ω-3 polyunsaturated fatty acids in fish lipids. J. Am Oil Chem Soc. 70, 225-228 (1993).
- Igarashi, T., Aursand, M., Hirata, Y., Gribbestad, I. S., Wada, S., Nonaka, M. Nondestructive quantitative acid and n-3 fatty acids in fish oils by high-resolution 1H nuclear magnetic resonance spectroscopy. J. Am. Oil Chem. Soc. 77, 737-748 (2000).
- Plans, M., Wenstrup, M., Saona, L. Application of Infrared Spectroscopy for Characterization Dietary Omega-3 Oil Supplements. J. Am. Oil Chem. Soc. 92, 957-966 (2015).
- Jian-hua, C. I. A. Near-infrared Spectrum Detection of Fish Oil DHA Content Based on Empirical Mode Decomposition and Independent Component Analysis. J Food Nutr Res. 2 (2), 62-68 (2014).
- Millen, A. E., Dodd, K. W., Subar, A. F. Use of vitamin, mineral, nonvitamin, and nonmineral supplements in the United States: The 1987, 1992, and 2000 National Health Interview Survey results. J. of Am. Diet Assoc. 104 (6), 942-950 (2004).
- Dwyer, J. T., et al. Progress in developing analytical and label-based dietary supplement databases at the NIH office of dietary supplements. J. Food Compos. Anal. 21, S83-S93 (2008).
- Monakhova, Y. B., Ruge, I., Kuballa, T., Lerch, C., Lachenmeier, D. W. Rapid determination of coenzyme Q10 in food supplements using 1H NMR spectroscopy. Int. J. Vitam. Nutr. Res. 83 (1), 67-72 (2013).
- Monakhova, Y. B., et al. Standardless 1H NMR determination of pharmacologically active substances in dietary supplements and medicines that have been illegally traded over the internet. Drug Test. Anal. 5 (6), 400-411 (2013).
- Berger, S., Braun, S. . 200 and more NMR experiments: a practical course. , (2004).
- Knothe, G., Kenar, J. A. Determination of the fatty acid profile by 1H-NMRspectroscopy. Eur. J. Lipid Sci. Technol. 106, 88-96 (2004).
- Sacchi, R., Medina, J. I., Aubourg, S. P., Paolillo, I. G. L., Addeo, F. Quantitative High-Resolution 13C NMR Analysis of Lipids Extracted from the White Muscle of Atlantic Tuna (Thunnus alalunga). J. Agric. Food Chem. 41 (8), 1247-1253 (1993).
- Dais, P., Misiak, M., Hatzakis, E. Analysis of marine dietary supplements using NMR spectroscopy. Anal. Methods. 7 (12), 5226-5238 (2015).
- Pickova, J., Dutta, P. C. Cholesterol Oxidation in Some Processed Fish Products. J. Anal. Oil Chem. Soc. 80 (10), 993-996 (2003).
- Siddiqui, N., Sim, J., Silwood, C. J. L., Toms, H., Iles, R. A., Grootveld, M. Multicomponent analysis of encapsulated marine oil supplements using high-resolution 1H and 13C NMR techniques. J. of Lipid Rsrch. 44 (12), 2406-2427 (2003).
- Sua´rez, E. R., Mugford, P. F., Rolle, A. J., Burton, I. W., Walter, J. A., Kralovec, J. A. 13C-NMR Regioisomeric Analysis of EPA and DHA in Fish Oil Derived Triacylglycerol Concentrates. J. Am. Oil Chem. Soc. 87, 1425-1433 (2010).
- Youlin, X. A., Moran, S., Nikonowiczband, E. P., Gao, X. Z-restored spin-echo 13C 1D spectrum of straight baseline free of hump, dip and roll. Magn. Reson. Chem. 46, 432-435 (2008).
- Tengku-Rozaina, T. M., Birch, E. J. Positional distribution of fatty acids on hoki and tuna oil triglycerides by pancreatic lipase and 13C NMR analysis. Eur. J. Lipid Sci. Technol. 116 (3), 272-281 (2014).
- Berry, S. E. E. Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats:An overview and implications for cardiovascular disease. Nutr. Res. Rev. 22 (1), 3-17 (2009).
- Hunter, J. E. Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids. 36, 655-668 (2001).
- Vlahov, G. Regiospecific analysis of natural mixtures of triglycerides using quantitative 13C nuclear magnetic resonance of acyl chain carbonyl carbons. Magnetic Res. in Chem. 36, 359-362 (1998).

